Summary: Direct carotid puncture is a safe and effective alternative to thrombectomy for stroke patients.
Source: Yale
Yale researchers have found ways to treat stroke patients that may otherwise be untreatable.

Chemical space contains every possible chemical compound. It includes every drug and material we know and every one we’ll find in the future. It’s practically infinite and can be frustratingly complex. That’s why some chemists are turning to artificial intelligence: AI can explore chemical space faster than humans, and it might be able to find molecules that would elude even expert scientists. But as researchers work to build and refine these AI tools, many questions still remain about how AI can best help search chemical space and when AI will be able to assist the wider chemistry community.
Outer space isn’t the only frontier curious humans are investigating. Chemical space is the conceptual territory inhabited by all possible compounds. It’s where scientists have found every known medicine and material, and it’s where we’ll find the next treatment for cancer and the next light-absorbing substance for solar cells.
But searching chemical space is far from trivial. For one thing, it might as well be infinite. An upper estimate says it contains 10180 compounds, more than twice the magnitude of the number of atoms in the universe. To put that figure in context, the CAS database—one of the world’s largest—currently contains about 108 known organic and inorganic substances, and scientists have synthesized only a fraction of those in the lab. (CAS is a division of the American Chemical Society, which publishes C&EN.) So we’ve barely seen past our own front doorstep into chemical space.
However, it was unclear how TERRA got to the tip of chromosomes and remained there. “The telomere makes up only a tiny bit of the total chromosomal DNA, so the question is ‘how does this RNA find its home?’” Lingner says. To address this question, postdoc Marianna Feretzaki and others in the teams of Joachim Lingner at EPFL and Lumir Krejci at Masaryk University set out to analyze the mechanism through which TERRA accumulates at telomeres, as well as the proteins involved in this process. The findings are published in * Nature*.
**Finding home**
By visualizing TERRA molecules under a microscope, the researchers found that a short stretch of the RNA is crucial to bring it to telomeres. Further experiments showed that once TERRA reaches the tip of chromosomes, several proteins regulate its association with telomeres. Among these proteins, one called RAD51 plays a particularly important role, Lingner says.
RAD51 is a well-known enzyme that is involved in the repair of broken DNA molecules. The protein also seems to help TERRA stick to telomeric DNA to form a so-called “RNA-DNA hybrid molecule”. Scientists thought this type of reaction, which leads to the formation of a three-stranded nucleic acid structure, mainly happened during DNA repair. The new study shows that it can also happen at chromosome ends when TERRA binds to telomeres. “This is paradigm-shifting,” Lingner says.
The researchers also found that short telomeres recruit TERRA much more efficiently than long telomeres. Although the mechanism behind this phenomenon is unclear, the researchers hypothesize that when telomeres get too short, either due to DNA damage or because the cell has divided too many times, they recruit TERRA molecules. This recruitment is mediated by RAD51, which also promotes the elongation and repair of telomeres. “TERRA and RAD51 help to prevent accidental loss or shortening of telomeres,” Lingner says. “That’s an important function.””
Molecules that accumulate at the tip of chromosomes are known to play a key role in preventing damage to our DNA. Now, researchers at EPFL have unraveled how these molecules home in on specific sections of chromosomes—a finding that could help to better understand the processes that regulate cell survival in aging and cancer.

Researchers know how to make precise genetic changes within the genomes of crops, but the transformed cells often refuse to grow into plants. One team has devised a new solution.
Scientists who want to improve crops face a dilemma: it can be difficult to grow plants from cells after you’ve tweaked their genomes.
A new tool helps ease this process by coaxing the transformed cells, including those modified with the gene-editing system CRISPR-Cas9, to regenerate new plants. Howard Hughes Medical Institute Research Specialist Juan M. Debernardi and Investigator Jorge Dubcovsky, together with David Tricoli at the University of California, Davis Plant Transformation Facility, Javier Palatnik from Argentina, and colleagues at the John Innes Centre, collaborated on the work. The team reports the technology, developed in wheat and tested in other crops, October 12, 2020, in the journal Nature Biotechnology.
“The problem is that transforming a plant is still an art,” Dubcovsky says. The success rate is often low – depending on the crop being modified, 100 attempts may yield only a handful of green shoots that can turn into full-grown plants. The rest fail to produce new plants and die. Now, however, “we have reduced this barrier,” says Dubcovsky, a plant geneticist at UC Davis. Using two genes that already control development in many plants, his team dramatically increased the formation of shoots in modified wheat, rice, citrus, and other crops.
Robots are now assisting in advancing developmental biology.
The study of developmental biology is getting a robotic helping hand.
Scientists are using a custom robot to survey how mutations in regulatory regions of the genome affect animal development. These regions aren’t genes, but rather stretches of DNA called enhancers that determine how genes are turned on and off during development. The team describes the findings—and the robot itself—on October 14 in the journal Nature.
“The real star is this robot,” says David Stern, a group leader at HHMI’s Janelia Research Campus. “It was extremely creative engineering.”

Partnership will use diagnostic imaging tools to explore health issues associated with microgravity, and apply this knowledge to patients on Earth.
The French Society of Radiology (SFR) and the country’s national centre for space exploration (CNES) have signed a partnership, details of which were streamed live at the Journées Francophones de Radiologie (JFR) congress on 4 October. The aim is to develop imaging solutions to be sent on space flights and to collaborate on image collection and optimization, teleradiology and training of astronauts.
France has the largest space program in Europe and the third oldest institutional space programme in history, along with Russia and the US. CNES, which has a long track record in space exploration, recognizes the great potential of diagnostic imaging for monitoring astronauts’ health while on missions, according to general director Lionel Suchet.
The plan is to create a “two-way street” in which radiologists and space experts will collaborate on innovative projects to make further progress, JFR delegates heard online at the plenary Antoine Béclère lecture. A SFR–CNES working group will now define the research themes and establish a schedule of tasks ahead by December.
Summary: By fusing a cytokine to a blood protein, researchers have developed a new therapy to help treat multiple sclerosis.
Source: University of Chicago
Multiple sclerosis, an autoimmune disease of the central nervous system that affects millions worldwide, can cause debilitating symptoms for those who suffer from it.


Eli Lilly (NYSE: LLY) reportedly paused a clinical trial testing its COVID-19 antibody treatment candidate because of a “potential safety concern.”
The New York Times reported that Eli Lilly’s testing site researchers were notified of the pause by emails sent by government officials (it is a government-sponsored trial) and the company later confirmed it. A spokesperson from the company told The Hill that “Safety is of the utmost importance to Lilly. We are aware that, out of an abundance of caution, the ACTIV-3 independent data safety monitoring board (DSMB) has recommended a pause in enrollment.”
Eli Lilly’s trial was comparing its therapy to a placebo, while all study participants also received the experimental drug remdesivir, which has been used in treating COVID-19 throughout the pandemic. The company’s therapeutic uses monoclonal antibodies in an effort to block the virus from infecting cells.
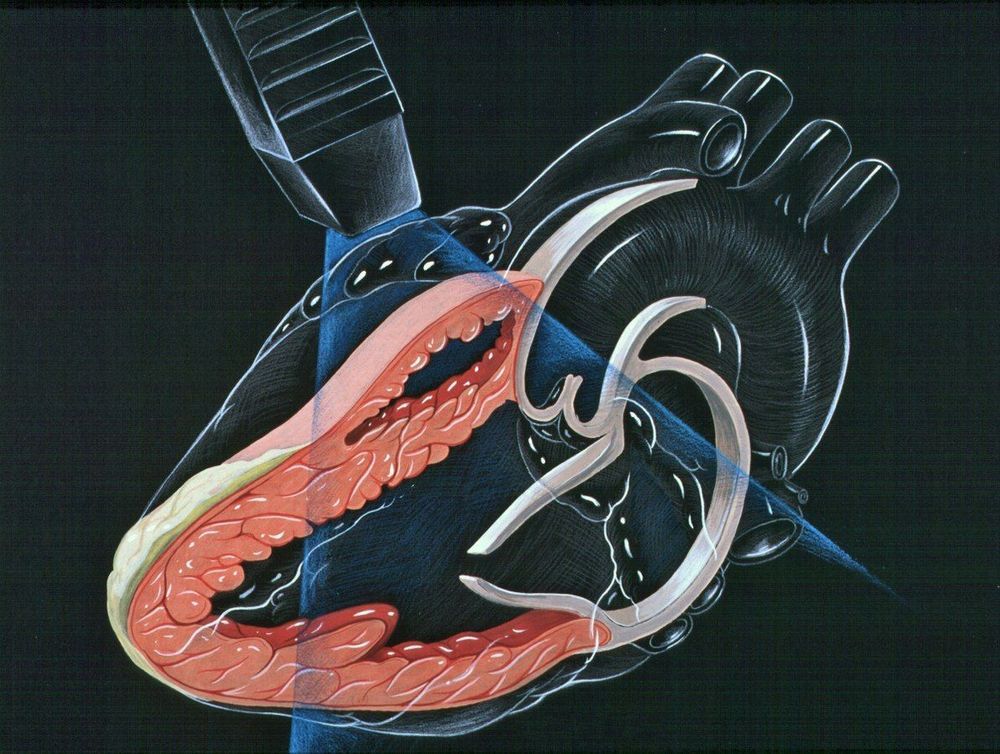
GE Healthcare has received 510k clearance from US FDA for its Ultra Edition package on Vivid cardiovascular ultrasound systems, which come with features based on artificial intelligence (AI) that allows clinicians to get quicker and more exams repeatedly. Although methodical evaluations of heart function are necessary in echocardiography, such evaluations can be time-consuming and difficult to get. Quality acquisition of data and operator skill are essential factors to get precise and thorough exams. Given that patients undergo subsequent monitoring exams, the reproducibility of the exam evaluations is essential to monitoring improvement or progress of the disease.