UMD researchers uncover key mechanisms in gene regulation that may lead to better design of RNA-based medicines.


Evan groover2024 NAPB borlaug scholargrad student — plant and microbial biology, UC berkeley.


Uniquely human features of neocortical development and maturation are not only intriguing for their implications in human-specific cognitive abilities, but they are also vulnerable to dysregulation which could cause or contribute to distinctly human brain disorder pathophysiology. The human cerebral cortex is essential for both cognition and emotional processing and dysregulation of these processes of the cortex are associated with a wide range of brain disorders including schizophrenia (SZ), autism spectrum disorder (ASD), Parkinson’s disease (PD), and Alzheimer’s disease (AD) (Berman and Weinberger, 1991; Rubenstein, 2011; Xu et al., 2019). Much remains to be learned about the mechanisms governing cortical expansion and responses to pathogenesis between human and non-human primates (NHPs) (Otani et al., 2016). Understanding these differences could shed light on the underlying mechanisms responsible for human-specific brain disorders and lead to the identification of key targets for the development of effective therapies.
Subtle differences observed by comparing human neurodevelopment to that of our closest evolutionary relatives could reveal underlying mechanisms, including genomic or transcriptional differences, contributing to varied phenotypes (Pollen et al., 2019). Human-specific responses to pathogenesis might be elucidated in a similar manner; by comparing brain pathophysiology of humans to our non-human primate counterparts (Hof et al., 2004). Although rodent models have taught us much about basic mammalian brain development and disorders (Fernando and Robbins, 2011), comparing governing processes and responses to species more closely related to humans can reduce the number of variables allowing for the identification of specific mechanisms responsible for observed deviations. Studies analyzing induced pluripotent stem cells (iPSCs) derived from humans, chimpanzees, and bonobos (Pan paniscus) show large sets of differentially expressed genes between human and NHP iPSCs. Perhaps the most compelling differentially expressed genes are those related to increased long interspersed element-1 (LINE-1) mobility in chimpanzees and bonobos, which could have implications on the rates of genetic divergence among species, and alternative mechanisms of pluripotency maintenance in chimpanzees (Marchetto et al., 2013; Gallego Romero et al., 2015). Furthermore, when human and NHP iPSCs were differentiated to neurons, they displayed distinctive migratory patterns at the neural progenitor cell (NPC) stage followed by contrasting morphology and timing of maturation in neurons (Marchetto et al., 2019). Despite the ability of two-dimensional (2D) PSC-derived neural cultures to demonstrate basic organization and transcriptomic changes of early brain development (Yan et al., 2013), while retaining the genetic background of the somatic cells from which they are reprogrammed, they lack the ability to develop complex cytoarchitecture, recapitulate advanced spatiotemporal transcriptomics, and brain region interconnectivity (including migration and axon guidance) of ensuing primate brain development (Soldner and Jaenisch, 2019). Intricate cellular heterogeneity, complex architecture, and interconnectivity of neurodevelopment, in addition to pathogenic responses, could be observed by comparing human and NHP brain tissues; however, ethical concerns and the inaccessibility of pre-and postnatal primate brain tissues limits the feasibility of such studies.
While brain organoids might be a long way from forming or sharing thoughts with us, they could still teach us much about ourselves. Brain organoids are three-dimensional (3D), PSC-derived structures that display complex radial organization of expanding neuroepithelium following embedding in an extracellular matrix like Matrigel and can recapitulate some subsequent processes of neurodevelopment including neurogenesis, gliogenesis, synaptogenesis, heterogenous cytoarchitecture, cell and axon migration, myelination of axons, and spontaneously-active neuronal networks (Lancaster et al., 2013; Bagley et al., 2017; Birey et al., 2017; Quadrato et al., 2017; Xiang et al., 2017; Marton et al., 2019; Shaker et al., 2021). It is likely that all these features of neurodevelopment are governed by some degree of specifies-specific dynamics. Brain organoids can be generated from human and NHP PSCs and, since some pathways regulating neural induction and brain region specification are well conserved in primates, both unguided cerebral organoids and guided brain region specific organoids can be generated (Mora-Bermúdez et al., 2016; Field et al., 2019; Kanton et al., 2019). Additional protocols have been established for the derivation of brain region specific organoids from human PSCs (hPSCs), including dorsal forebrain, ventral forebrain, midbrain, thalamus, basal ganglia, cerebellum, and telencephalic organoids (Muguruma et al., 2015; Sakaguchi et al., 2015; Jo et al., 2016; Bagley et al., 2017; Birey et al., 2017; Watanabe et al., 2017; Xiang et al., 2017, 2019; Qian et al., 2018). With some modifications, these methods could prove to be successful in establishing brain region-specific organoids from a variety of NHP PSC lines allowing for the reproducible comparison of homogeneous, human-specific neurodevelopment and brain disorder pathophysiology in brain regions beyond the cortex.

Has NASA canceled the SLS moon rocket? Will the Artemis Program be canceled too? Can we still beat China to the moon? See why that is vital and my role in helping to establish both the commercial space and SLS programs.
GoldBacks from Galactic/Green Greg’s affiliate link:
https://www.defythegrid.com/goldbacks… coupon code GreenGregs for 1% off Outstanding Antioxidant for Your Health: https://shopc60.com/ Use discount code: GreenGregs10 for 10% off Inspire your kids to love science! SAVE 20% OFF New Science Kits Using Code: NEWKITSSAVE20 https://www.pntra.com/t/SENKTExNSUhDR… For gardening in your Lunar or Mars habitat GalacticGregs has teamed up with True Leaf Market http://www.pntrac.com/t/TUJGRklGSkJGT… Awesome deals for long term food supplies for those long missions to deep space (or prepping in case your spaceship crashes: See the Special Deals at My Patriot Supply: www.PrepWithGreg.com For that off-grid asteroid homestead stock up with Lemans before you blast off: https://www.pntrs.com/t/SENJR0ZOSk9DR…
Scientists have translated nanoscale experimental and computational data into precise 3D representations of bacteria, yeast and human epithelial, breast and breast cancer cells in Minecraft, a video game that allows players to explore, build and manipulate structures in three dimensions.
The innovation will allow researchers and students of all ages to navigate biological cells, puncturing through the membranes of organelles to view their interiors or wandering across the cytoplasm to see how the various structures are distributed within the cell.
“CraftCells: A Window into Biological Cells” is the first broadly accessible tool allowing users to get an accurate picture of whole cells in 3D, said Zaida (Zan) Luthey-Schulten, a professor of chemistry and of physics at the University of Illinois Urbana-Champaign who led the work with Illinois bioengineering professors Stephen Boppart and Rohit Bhargava, graduate student Kevin Tan, postdoctoral researchers Zane Thornburg and Seth Kenkel, and study lead author Tianyu Wu, a biophysics graduate student at the U. of I.
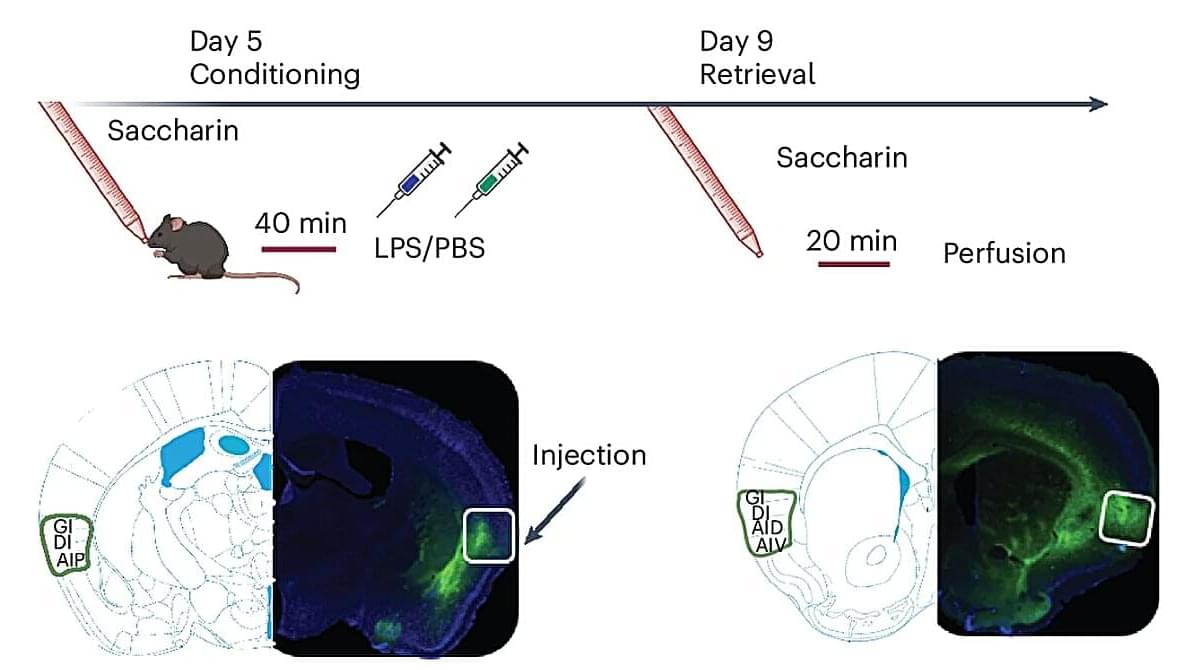
The brain of humans and other animals is known to contribute to the protection of the body from infections. Past studies have unveiled the existence of the so-called conditioned immune response (CIR), which is a form of Pavlovian conditioning that entails the formation of mental associations between specific sensory stimuli (e.g., a specific odor, taste, etc.) and immunomodulatory agents (i.e., a substance that influences the immune system).
For instance, if an animal tastes a particular food shortly before becoming ill several times, re-experiencing the same taste can evoke an aversive response and even trigger an anticipatory immune response. While this CIR is now well-documented, its neural underpinnings so far remain poorly understood.
Researchers at University of Haifa recently carried out a study aimed at better understanding the neural pathways involved in these widely reported conditioned immune responses (CIRs). Their paper, published in Nature Neuroscience, outlines a neural pathway that appears to mediate the retrieval of CIRs in male mice.