New insights on the Thalamo-Cortical Nuero Netowrk of Acute Ischaemic Stroke victims.
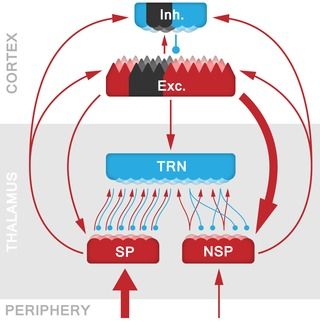
The neocortex and thalamus provide a core substrate for perception, cognition, and action, and are interconnected through different direct and indirect pathways that maintain specific dynamics associated with functional states including wakefulness and sleep. It has been shown that a lack of excitation, or enhanced subcortical inhibition, can disrupt this system and drive thalamic nuclei into an attractor state of low-frequency bursting and further entrainment of thalamo-cortical circuits, also called thalamo-cortical dysrhythmia (TCD). The question remains however whether similar TCD-like phenomena can arise with a cortical origin. For instance, in stroke, a cortical lesion could disrupt thalamo-cortical interactions through an attenuation of the excitatory drive onto the thalamus, creating an imbalance between excitation and inhibition that can lead to a state of TCD. Here we tested this hypothesis by comparing the resting-state EEG recordings of acute ischaemic stroke patients (N = 21) with those of healthy, age-matched control-subjects (N = 17). We observed that these patients displayed the hallmarks of TCD: a characteristic downward shift of dominant α-peaks in the EEG power spectra, together with increased power over the lower frequencies (δ and θ-range). Contrary to general observations in TCD, the patients also displayed a broad reduction in β-band activity. In order to explain the genesis of this stroke-induced TCD, we developed a biologically constrained model of a general thalamo-cortical module, allowing us to identify the specific cellular and network mechanisms involved. Our model showed that a lesion in the cortical component leads to sustained cell membrane hyperpolarization in the corresponding thalamic relay neurons, that in turn leads to the de-inactivation of voltage-gated T-type Ca2+ -channels, switching neurons from tonic spiking to a pathological bursting regime. This thalamic bursting synchronises activity on a population level through divergent intrathalamic circuits, and entrains thalamo-cortical pathways by means of propagating low-frequency oscillations beyond the restricted region of the lesion. Hence, pathological stroke-induced thalamo-cortical dynamics can be the source of diaschisis, and account for the dissociation between lesion location and non-specific symptoms of stroke such as neuropathic pain and hemispatial neglect.
The thalamus is involved in the relay and processing of most sensory information, and provides an interface between subcortical structures and the neocortex. However, disruptions in the subcortical communication with the thalamus are known to lead to thalamo-cortical dysrhythmia (TCD), which is linked to symptoms in a range of illnesses including Parkinson’s disease, neurogenic pain syndrome and tinnitus. Thus far, TCD has solely been interpreted in terms of changes within subcortical pathways, but here we investigate how cortical disturbances (i.e., ischaemic stroke) may affect thalamic function in a similar manner. We do so by analysing the electroencephalogram (EEG) of stroke patients with a cortical lesion, and show that their EEG power spectra display the characteristic features of TCD.
Continue reading “The Impact of Cortical Lesions on Thalamo-Cortical Network Dynamics after Acute Ischaemic Stroke: A Combined Experimental and Theoretical Study” »