Startup seeks new biomarkers as it develops cellular reprogramming drugs designed to reverse brain aging and combat dementia.

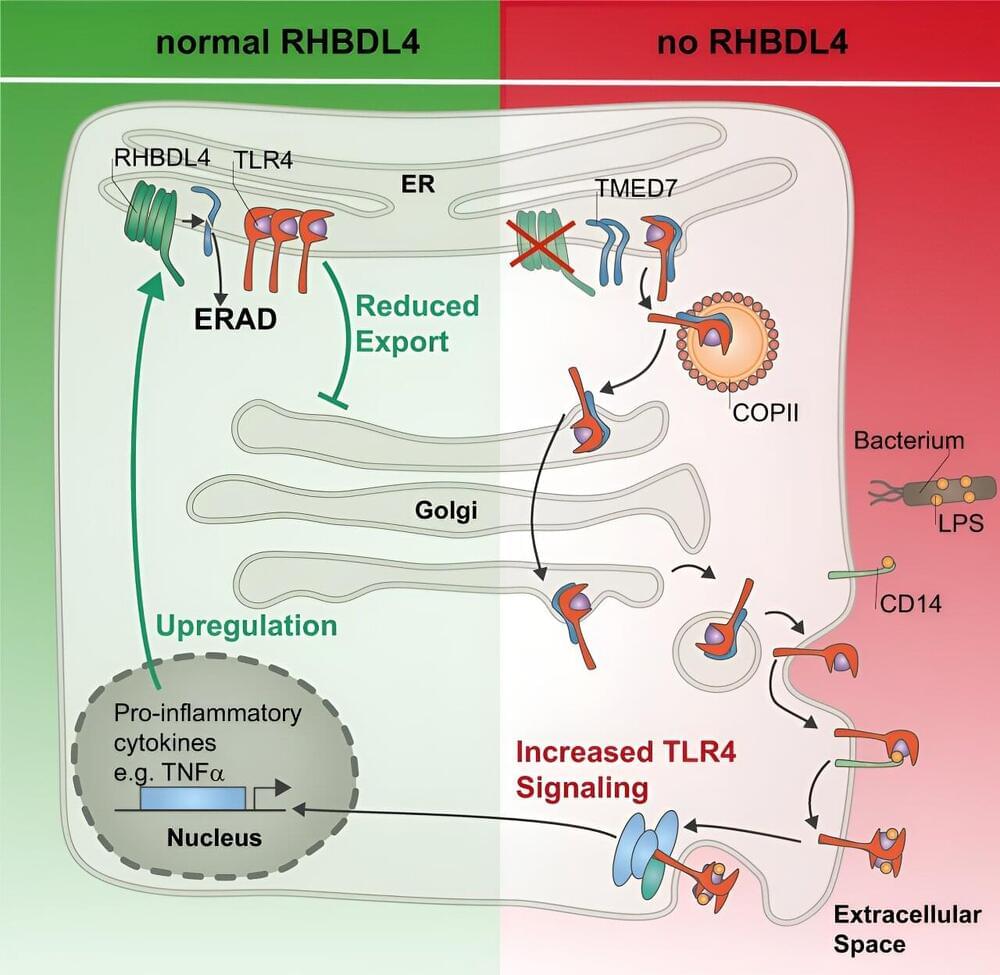
Researchers from the UoC’s Center for Biochemistry at the Faculty of Medicine and the UoC CECAD Cluster of Excellence in Aging Research have discovered that an excessive immune response can be prevented by the intramembrane protease RHBDL4.
In a study now published in Nature Communications under the title “RHBDL4-triggered downregulation of COPII adaptor protein TMED7 suppresses TLR4-mediated inflammatory signaling,” the previously unknown regulatory mechanism is described.
The researchers discovered that the cleavage of a cargo receptor by a so-called intramembrane protease reduces the localization of a central immune receptor on the cell surface and thereby the risk of an overreaction of the immune system.
Guanylate binding proteins (GBP) were discovered by YSM’s John MacMicking, PhD, and colleagues over a decade ago as major organizers of cellular immune response.
In a recent study, MacMicking’s team used advanced cryo-and electron microscope technology to visualize in high resolution the way GBPs…
Yale scientists have discovered a family of immune proteins, which they describe as a “massive molecular machine,” that could affect the way our bodies fight infection.
Our immune system mobilizes numerous proteins to detect viruses and bacteria — and to bring them under control. But until recently, limits to research technology have thwarted scientists’ understanding of how to prevent different pathogens from occupying and replicating within specific parts of our cells in the first place.
Harnessing the latest cryo‐electron microscopy techniques to look inside human cells, researchers at the Yale Systems Biology Institute have identified a family of large immune proteins that assemble into a massive signaling platform directly on the surface of microbial pathogens.
Bats are an important group of mammals to understand the ecology, diversity, and transmission of associated microbes – including viruses, bacteria, and fungi.
Over the past two decades, research on bat-associated microbes such as viruses, bacteria and fungi has dramatically increased. Here, we synthesize themes from a conference symposium focused on advances in the research of bats and their microbes, including physiological, immunological, ecological and epidemiological research that has improved our understanding of bat infection dynamics at multiple biological scales. We first present metrics for measuring individual bat responses to infection and challenges associated with using these metrics. We next discuss infection dynamics within bat populations of the same species, before introducing complexities that arise in multi-species communities of bats, humans and/or livestock. Finally, we outline critical gaps and opportunities for future interdisciplinary work on topics involving bats and their microbes.
Studies of bat-associated microbes (i.e. microorganisms detected in or isolated from bats) date back to rabies virus investigations in the early 1900s [1]. In the past two decades, following the emergence of Severe Acute Respiratory Syndrome (SARS) coronavirus (CoV) in 2003 and SARS-CoV-2 in 2019, there has been a dramatic increase in research on bat-associated microbes, including viruses, bacteria, haemosporidians and fungi [2–5]. These microbes may or may not cause disease in bats, and thus we broadly use the term ‘microbes’ rather than ‘pathogens’ throughout this paper to acknowledge that detecting microorganisms in bats is distinct from the process of determining pathogenicity [6].
Emerging nanotechnology and molecular innovations present promising strategies in combating inflammation and diabetes, aiming to transform treatment methods and improve patient outcomes significantly.
The intersection of nanotechnology and biomedicine has sparked significant advances in the treatment and understanding of both inflammatory and metabolic diseases. These advances have brought about innovative solutions to longstanding medical challenges, such as rheumatoid arthritis (RA) and type 2 diabetes mellitus (T2DM), diseases that collectively affect millions worldwide.

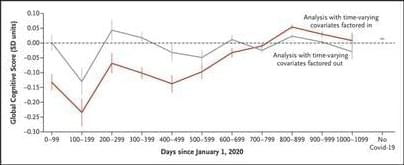
Summary: A recent study showcases a significant leap in the study of brain oscillations, particularly ripples, which are crucial for memory organization and are affected in disorders like epilepsy and Alzheimer’s. Researchers have developed a toolbox of AI models trained on rodent EEG data to automate and enhance the detection of these oscillations, proving their efficacy on data from non-human primates.
This breakthrough, stemming from a collaborative hackathon, offers over a hundred optimized machine learning models, including support vector machines and convolutional neural networks, freely available to the scientific community. This development opens new avenues in neurotechnology applications, especially in diagnosing and understanding neurological disorders.
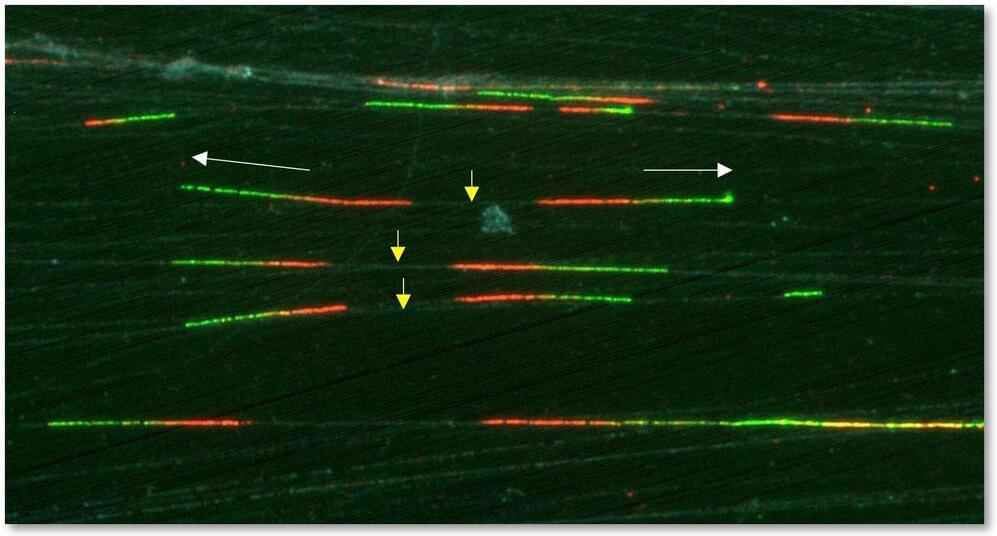
Every time a cell divides, its DNA is duplicated so that the two daughter cells have the same genetic material as their parent. This means that, millions of times a day, a biochemical wonder takes place in the body: the copying of the DNA molecule. It is a high-precision job carried out by specific proteins and includes systems to protect against potential errors that could lead to diseases such as cancer.
One of these anti-failure systems has just been discovered by researchers in the DNA Replication Group at the Spanish National Cancer Research Centre (CNIO), led by Juan Méndez. It is based on a protein that ensures that DNA is copied only once, as it should be, and not twice or more.
The work is published in The EMBO Journal.

Generative AI is getting plenty of attention for its ability to create text and images. But those media represent only a fraction of the data that proliferate in our society today. Data are generated every time a patient goes through a medical system, a storm impacts a flight, or a person interacts with a software application.
Using generative AI to create realistic synthetic data around those scenarios can help organizations more effectively treat patients, reroute planes, or improve software platforms—especially in scenarios where real-world data are limited or sensitive.
For the last three years, the MIT spinout DataCebo has offered a generative software system called the Synthetic Data Vault to help organizations create synthetic data to do things like test software applications and train machine learning models.
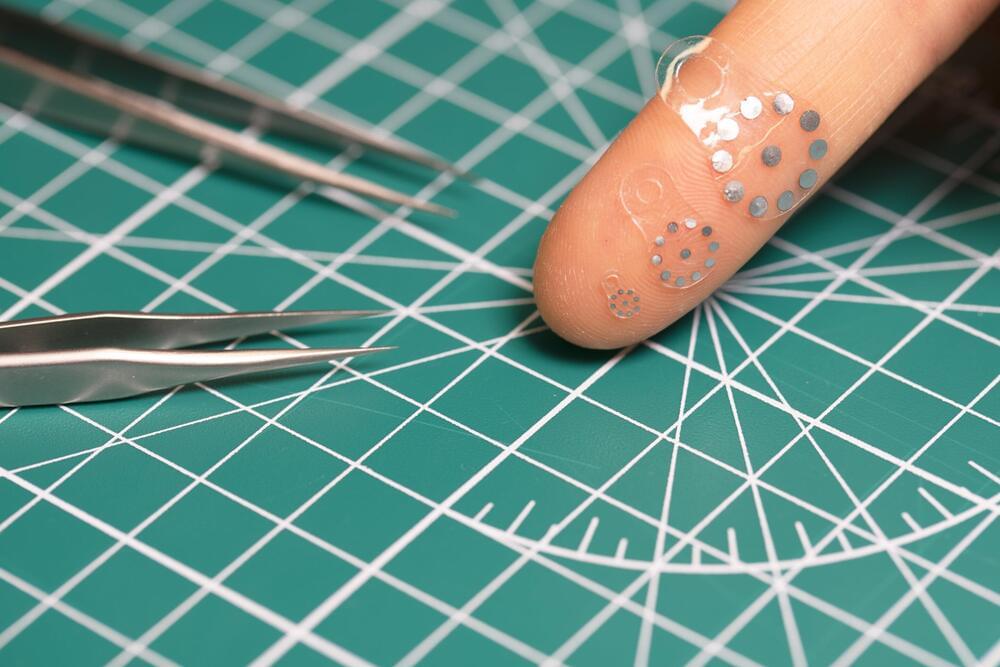
Researchers led by Northwestern University and Washington University School of Medicine in St. Louis have developed a new, first-of-its-kind sticker that enables clinicians to monitor the health of patients’ organs and deep tissues with a simple ultrasound device.
When attached to an organ, the soft, tiny sticker changes in shape in response to the body’s changing pH levels, which can serve as an early warning sign for post-surgery complications such as anastomotic leaks. Clinicians then can view these shape changes in real time through ultrasound imaging.
Currently, no existing methods can reliably and non-invasively detect anastomotic leaks—a life-threatening condition that occurs when gastrointestinal fluids escape the digestive system. By revealing the leakage of these fluids with high sensitivity and high specificity, the non-invasive sticker can enable earlier interventions than previously possible. Then, when the patient has fully recovered, the biocompatible, bioresorbable sticker simply dissolves away—bypassing the need for surgical extraction.