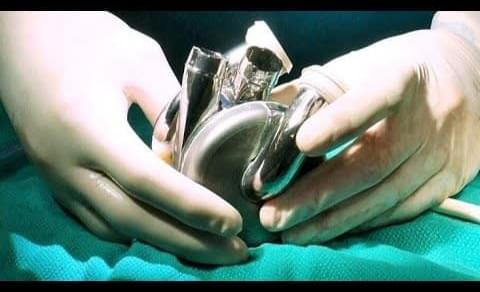
The Texas Heart Institute (THI) and BiVACOR®, a clinical-stage medical device company, announced today the successful first-in-human implantation of the BiVACOR Total Artificial Heart (TAH) as part of the U.S. Food and Drug Administration (FDA) Early Feasibility Study (EFS) on July 9, 2024.
BiVACOR’s TAH is a titanium-constructed biventricular rotary blood pump with a single moving part that utilizes a magnetically levitated rotor that pumps the blood and replaces both ventricles of a failing heart.









Leave a reply