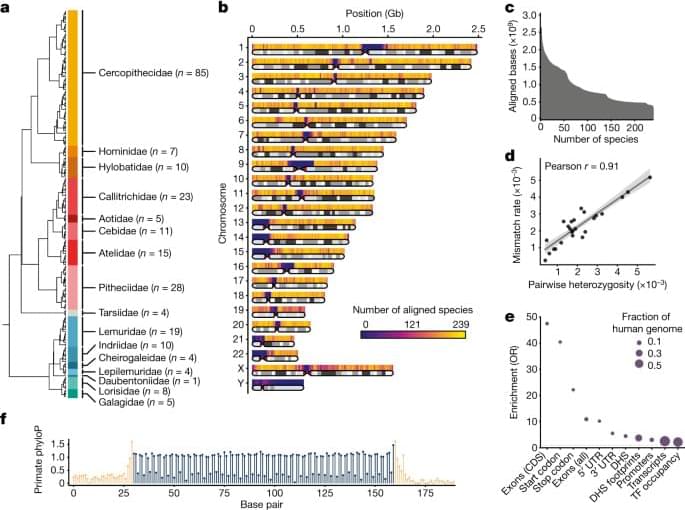
A tiny but prolific world of microbes encompasses everything around us, both inside and out. Microbiomes, which are comprised of diverse communities of microbes, play a pivotal role in shaping human health, yet the intricacies of how different microbial compositions influence our well-being remain largely unknown.
In a recent study published in Proceedings of the National Academy of Sciences, researchers at the University of Illinois Urbana-Champaign describe a new framework they have created to predict how species within microbiomes interact with each other to create unique compositions.
“Microbes can be used in medicine, aka ‘bugs as drugs,’ and these microbial therapeutics hold the possibility of being the answer to many of the diseases we face today,” said Shreya Arya, a graduate student in the O’Dwyer lab.