The hand could lead to advanced prosthetic hands for amputees or robots with the dexterity and strength to perform household task.
Category: biotech/medical
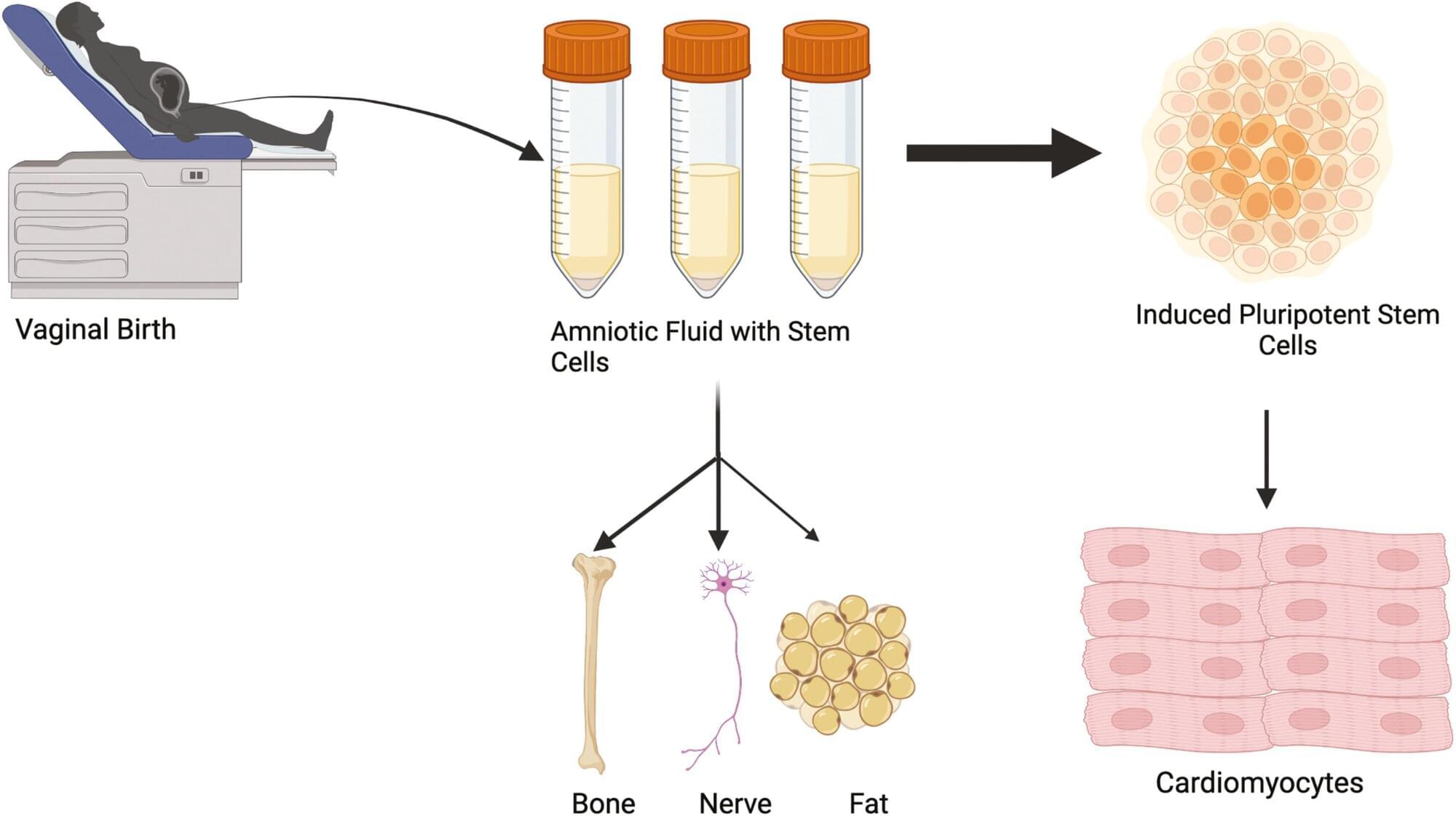
Amniotic stem cells can be collected from vaginal fluid rather than more invasive techniques
Researchers at the University of Colorado Anschutz Medical Campus have discovered that amniotic fluid stem cells can be safely collected from vaginal fluid after childbirth rather than relying on more invasive methods that can pose some risk to the mother and fetus.
“We can then turn those cells into beating heart cells and use them later in treating congenital heart defects,” said the study’s senior author Jeffrey Jacot, Ph.D., associate professor of pediatrics and bioengineering at the University of Colorado Center for Bioengineering in the CU School of Medicine. “These results allow for an expanded and readily available source of amniotic stem cells beyond traditional collection through amniocentesis.”
The study was published today in the journal Stem Cells Translational Medicine.
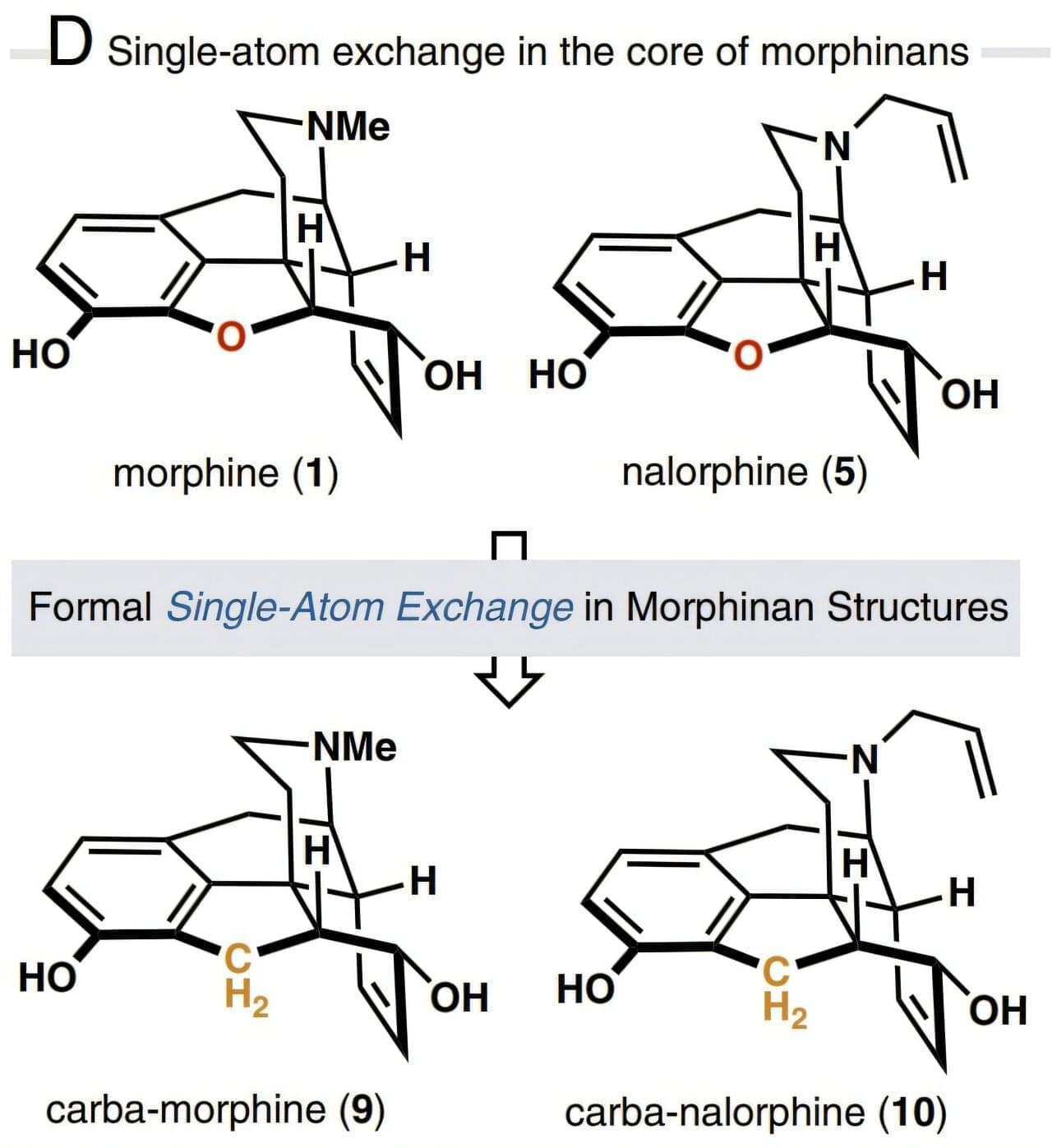
Atomic swap in morphine core structure leads to safer, non-rewarding opioid alternative
One of the greatest revolutions in the field of pain medication was the isolation of morphine from the opium poppy in the 19th century. Morphine molecules act as painkillers by attaching themselves to the µ-opioid receptor (MOR) in the central nervous system and blocking the brain from sending pain signals to the rest of the body. This potent opioid analgesic also has side effects such as constipation, respiratory depression, and even serious addiction problems.
A new study published in the Proceedings of the National Academy of Sciences has found that a single heavy atom replacement in the morphine core structure can transform its pharmacological behavior, resulting in reduced respiratory depression and no evidence of reward behavior—a key component of addiction tendencies—even at high doses.
Based on the insight that core-atom changes to the opioid drug molecule may exhibit biological effects distinct from the parent compound, the researchers developed a 15-step total synthesis of a morphine derivative where an oxygen atom in the E-ring is replaced with a methylene (CH2) group and called the new derivative carbamorphine.

Theory for aerosol droplets from contaminated bubbles may shed light on spread of pollution, microplastics, and more
Bubbles burst when their caps rupture. Children discover this phenomenon every summer day, but it also underpins key mechanisms for the spread of pollutants, contaminants, and even infectious disease through the generation of aerosol droplets. While bubble bursting has been extensively studied in pure substances, the impact of contaminants on bursting dynamics has not received widespread attention.
Researchers in The Grainger College of Engineering at the University of Illinois Urbana-Champaign have conducted a systematic study to investigate bubble-bursting jets—aerosol particles sprayed when bubble surfaces rupture—when surface contaminants are present. The laboratory of mechanical science and engineering professor Jie Feng developed a model predicting the influence of contaminants on jet size and experimentally confirmed it.
The study is published in the journal Physical Review Letters, where it was selected as an Editors’ Suggestion.
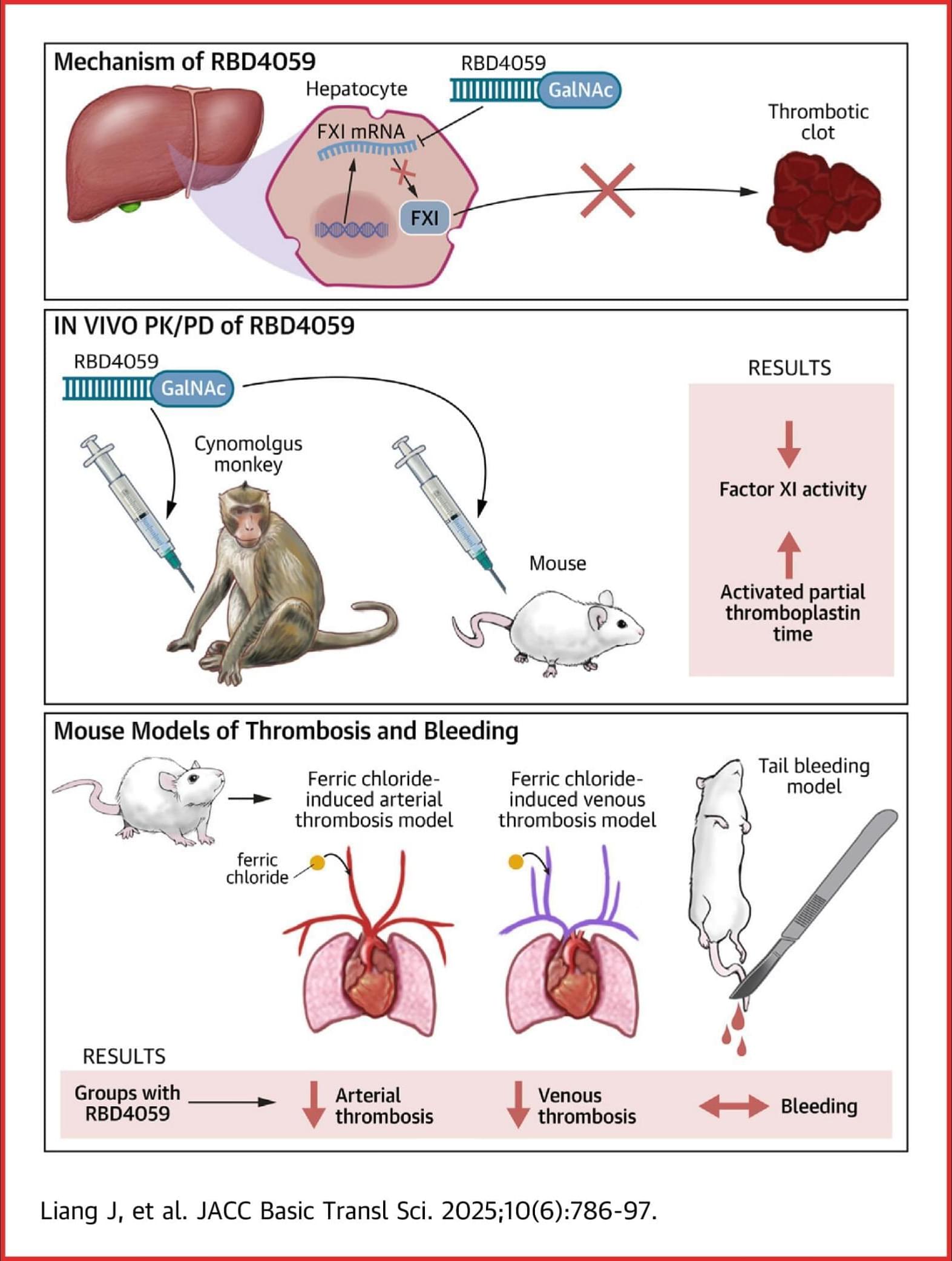
A Novel GalNAc-siRNA With Potent and Durable Antithrombotic Effects
In the present study, the GalNAc-siRNA molecule RBD4059 showed promising reduction of FXI activity in preclinical animal models and antithrombotic effects without disrupting hemostasis.
RBD4059 is the first FXI-targeting GalNAc-siRNA molecule to reach the clinical stage of development and is a promising candidate for the development of a safe and efficient antithrombotic drug with high patient compliance.


‘I was floored by the data’: Psilocybin shows anti-aging properties in early study
Psilocybin, the main psychoactive ingredient in magic mushrooms, extends the lifespan of human cells, a lab study suggests. Researchers also found that the psychedelic compound slows certain hallmarks of aging in older mice while improving their fur quality.
The findings, published July 8 in the journal npj Aging, provide the first experimental evidence of psilocybin’s potential anti-aging properties.

Antibody sIgM emerges as a key guardian of gut health and metabolism
A pioneering new study published in Nature Microbiology, led by J. Oriol Sunyer, professor of immunology and pathobiology at the School of Veterinary Medicine, and a team of researchers at Penn Vet and the University of New Mexico, has uncovered a surprising new player in gut health: an antibody called secretory immunoglobulin M (sIgM).
While another antibody, secretory immunoglobulin A (sIgA), has long been known for helping balance the bacteria in our intestines, this new research shows that sIgM may be just as vital—if not more so—in protecting gut health and maintaining overall well-being.
Secretory immunoglobulins—immunoglobulins found in the mucosal surfaces or linings of various organs and tracts of vertebrates—modulate the colonization, composition, and metabolism of the gut microbiome. While sIgA and secretory immunoglobulin T (sIgT) are considered the key immunoglobulins involved in the maintenance of microbiome homeostasis in the gut of mammals and fish, respectively, Sunyer and his colleagues challenged this paradigm by demonstrating that sIgM plays a crucial and non-redundant role in the regulation of gut microbiota and metabolism.
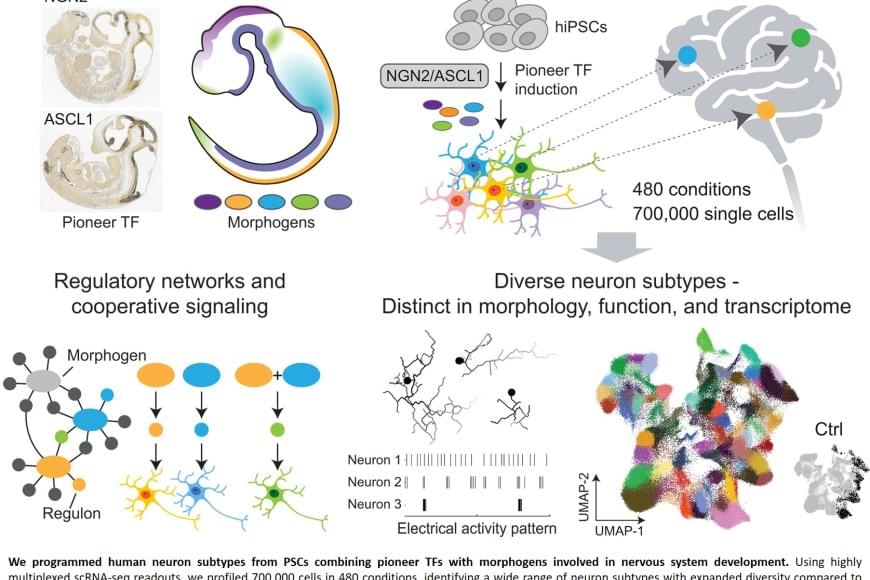
Producing library of heterogeneous human neurons from stem cells
Nerve cells are not just nerve cells. Depending on how finely we distinguish, there are several hundred to several thousand different types of nerve cell in the human brain according to the latest calculations. These cell types vary in their function, in the number and length of their cellular appendages, and in their interconnections. They emit different neurotransmitters into our synapses and, depending on the region of the brain – for example, the cerebral cortex or the midbrain – different cell types are active.
When scientists produced nerve cells from stem cells in Petri dishes for their experiments in the past, it was not possible to take their vast diversity into account. Until now, researchers had only developed procedures for growing a few dozen different types of nerve cell in vitro. They achieved this using genetic engineering or by adding signalling molecules to activate particular cellular signalling pathways. However, they never got close to achieving the diversity of hundreds or thousands of different nerve cell types that actually exists.
“Neurons derived from stem cells are frequently used to study diseases. But up to now, researchers have often ignored which precise types of neuron they are working with,” saysthe senior author. However, this is not the best approach to such work. “If we want to develop cell culture models for diseases and disorders such as Alzheimer’s, Parkinson’s and depression, we need to take the specific type of nerve cell involved into consideration.”