Scientists studying ability of mice to regenerate ear damage say therapies based on retinoic acid might work across various tissue types.


Join us on Patreon! https://www.patreon.com/MichaelLustgartenPhD
Discount Links/Affiliates:
Blood testing (where I get the majority of my labs): https://www.ultalabtests.com/partners/michaellustgarten.
At-Home Metabolomics: https://www.iollo.com?ref=michael-lustgarten.
Use Code: CONQUERAGING At Checkout.
Clearly Filtered Water Filter: https://get.aspr.app/SHoPY
Epigenetic, Telomere Testing: https://trudiagnostic.com/?irclickid=U-s3Ii2r7xyIU-LSYLyQdQ6…M0&irgwc=1
Use Code: CONQUERAGING
NAD+ Quantification: https://www.jinfiniti.com/intracellular-nad-test/

A new gene therapy delivery device could let hospital pharmacies make personalized nanomedicines to order. This democratized approach to precision medicine, as published in Frontiers in Science, could revolutionize how hospitals treat rare diseases, even in low-resource settings.
Rare diseases affect millions worldwide, yet the one-size-fits-all model of drug development leaves patients with few treatment options. Now a European research project called NANOSPRESSO aims to tip the balance in patients’ favor by boosting access to low-cost bespoke gene and RNA therapies.
The prototype NANOSPRESSO device combines two proven technologies— nucleic acid therapeutics and lipid nanoparticles—into a portable manufacturing unit. Hospital pharmacists could use the unit to prepare sterile, injectable nanomedicines tailored to the specific genetic abnormality causing the patient’s condition, bypassing the need for centralized drug production.
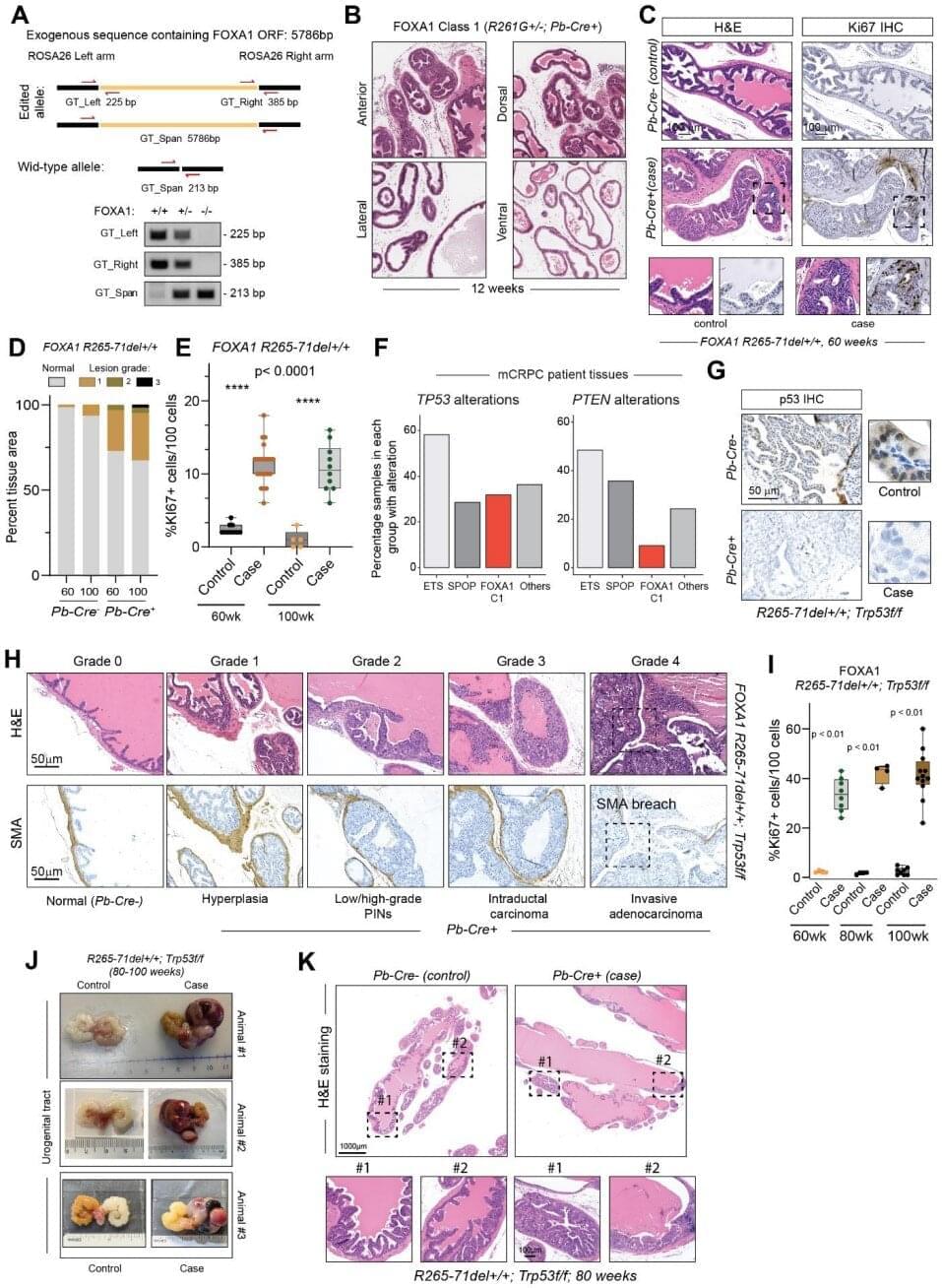
A new study from the University of Michigan Rogel Health Cancer Center, published in Science, sheds light on how two distinct classes of mutations in the FOXA1 gene—commonly altered in prostate cancer—drive tumor initiation formation and therapeutic resistance.
FOXA1, a key transcription factor that facilitates androgen receptor binding to DNA, is mutated in 10–40% of hormone-dependent prostate cancers. While common, the exact ways these mutations alter cancer cells have remained elusive—until now.
Rogel researchers, including Arul Chinnaiyan, M.D., Ph.D., S.P. Hicks Endowed Professor of Pathology and Urology, and Abhijit Parolia, Ph.D., Rogel Fellow and Assistant Professor of Pathology, used mouse models to understand the mechanisms underlying two major classes of FOXA1 mutations.

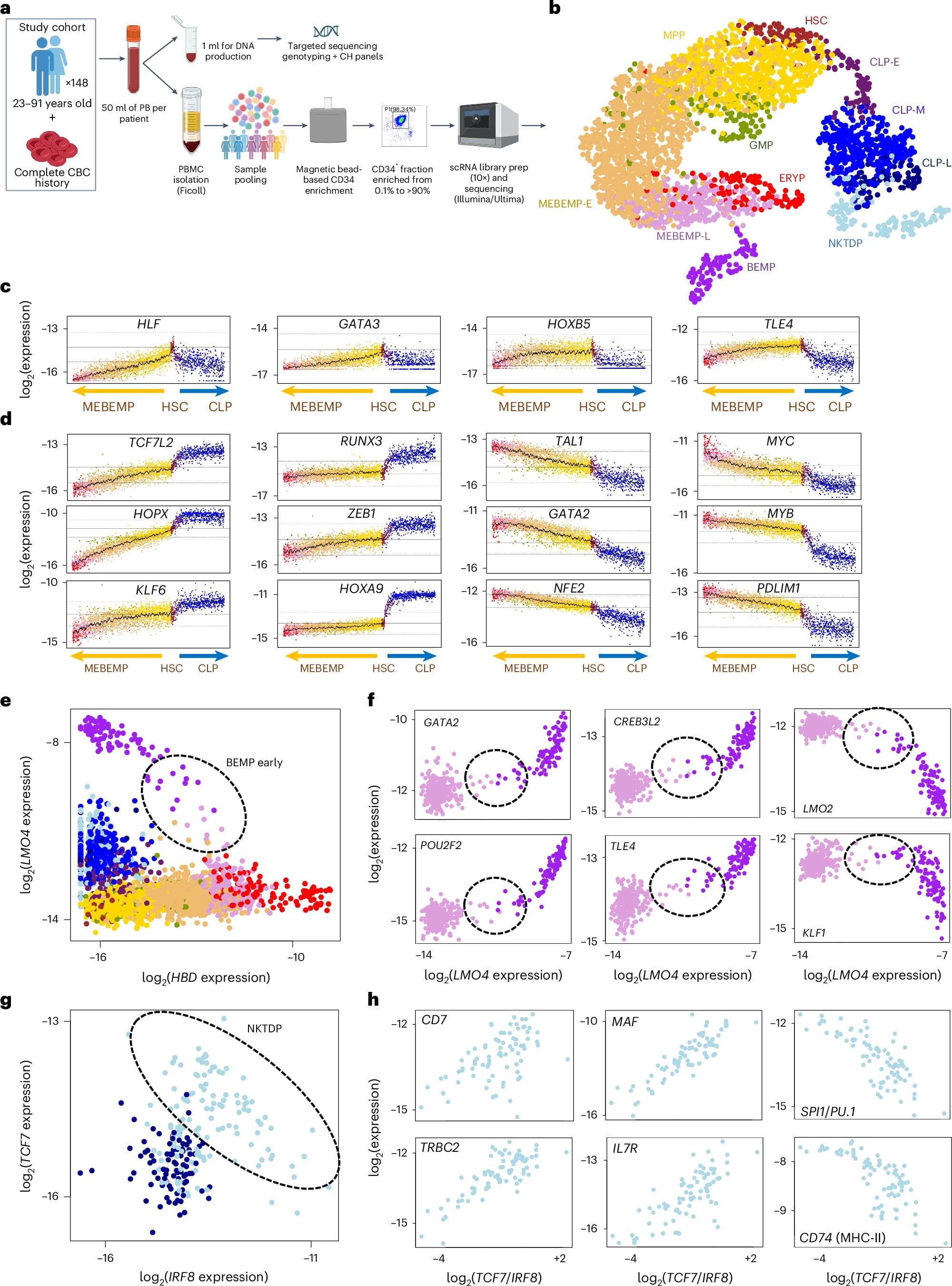
What if a blood test could reveal the pace of our aging—and the diseases that may lie ahead? The labs of Profs. Liran Shlush and Amos Tanay at the Weizmann Institute of Science have been conducting in-depth studies into the biology of blood to better understand the aging process and why some people become more susceptible to disease over the years.
Their research teams, made up of physicians, biologists and data scientists, have been tracking changes in the blood-forming stem cells, including the emergence of genetic changes in these cells in about one-third of people over the age of 40. These changes not only increase the risk of blood cancers such as leukemia, but have also been linked to heart disease, diabetes and other age-related conditions.
In a new study published today in Nature Medicine, Shlush and Tanay present findings that may lead to an innovative blood test for detecting a person’s risk of developing leukemia. This test may potentially replace the invasive diagnostic procedure of bone marrow sampling.
Gene therapy—once something out of science fiction—is now being used in real hospitals to treat real people. Gene editing has become a conversation of not only treating rare diseases but also about access, fairness, and how much control we should have over our biology.
Genes are sections of DNA that act like instruction manuals telling our cells how to build proteins. Proteins perform vital function like energy use, cellular communications, immunity and cell repair. So when people say “We are what our genes make us,” it’s because these gene-coded proteins guide our growth, health, and behaviour.
Sometimes, typos appear in these instruction manuals. They are called genetic mutations. While many mutations are harmless, some affect the protein made from the mutated gene and disrupt how the cell functions. Some cause serious diseases like cystic fibrosis, muscular atrophy or certain cancers.
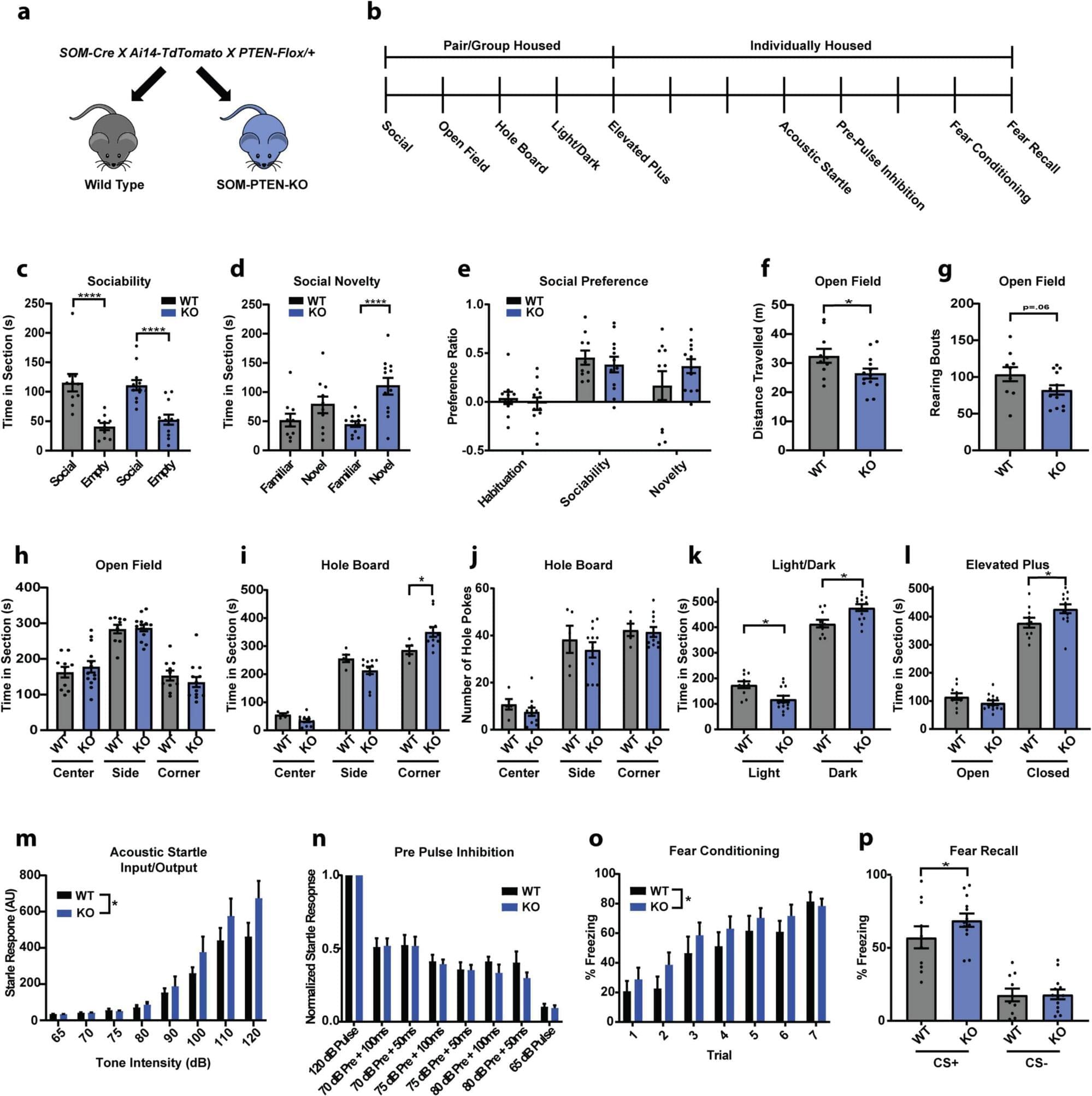
Researchers at the Max Planck Florida Institute for Neuroscience have discovered how loss of a gene strongly associated with autism and macrocephaly (large head size) rewires circuits and alters behavior.
Their findings, published in Frontiers in Cellular Neuroscience, reveal specific circuit changes in the amygdala resulting from PTEN loss in inhibitory neurons, providing new insights into the underlying circuit alterations that contribute to heightened fear and anxiety.
PTEN has emerged as one of the most significant autism risk genes. Variations in this gene are found in a significant proportion of people with autism who also exhibit brain overgrowth, making it a key player in understanding differences in brain function. To investigate the impact of PTEN misregulation, researchers have turned to animal models, where global reduction of PTEN results in altered sociability, repetitive behaviors, and increased anxiety that are often associated with ASD in humans.