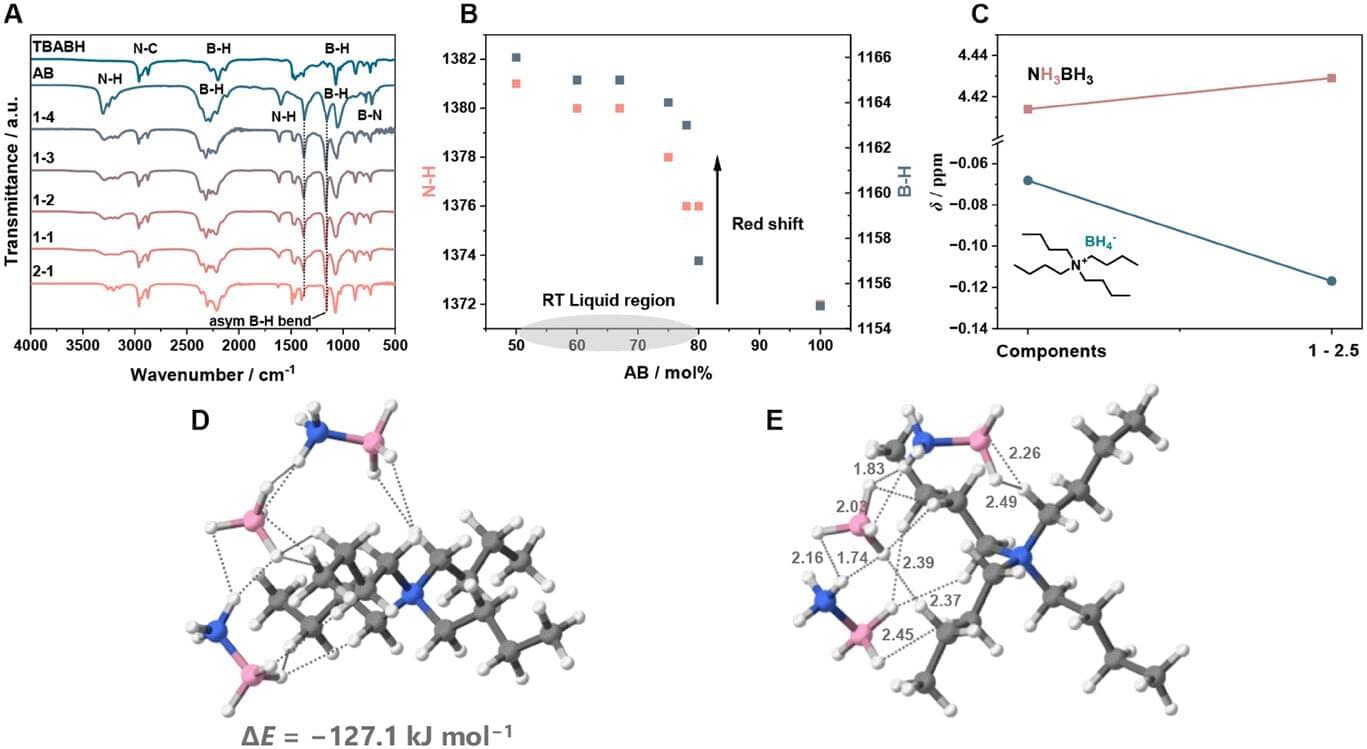
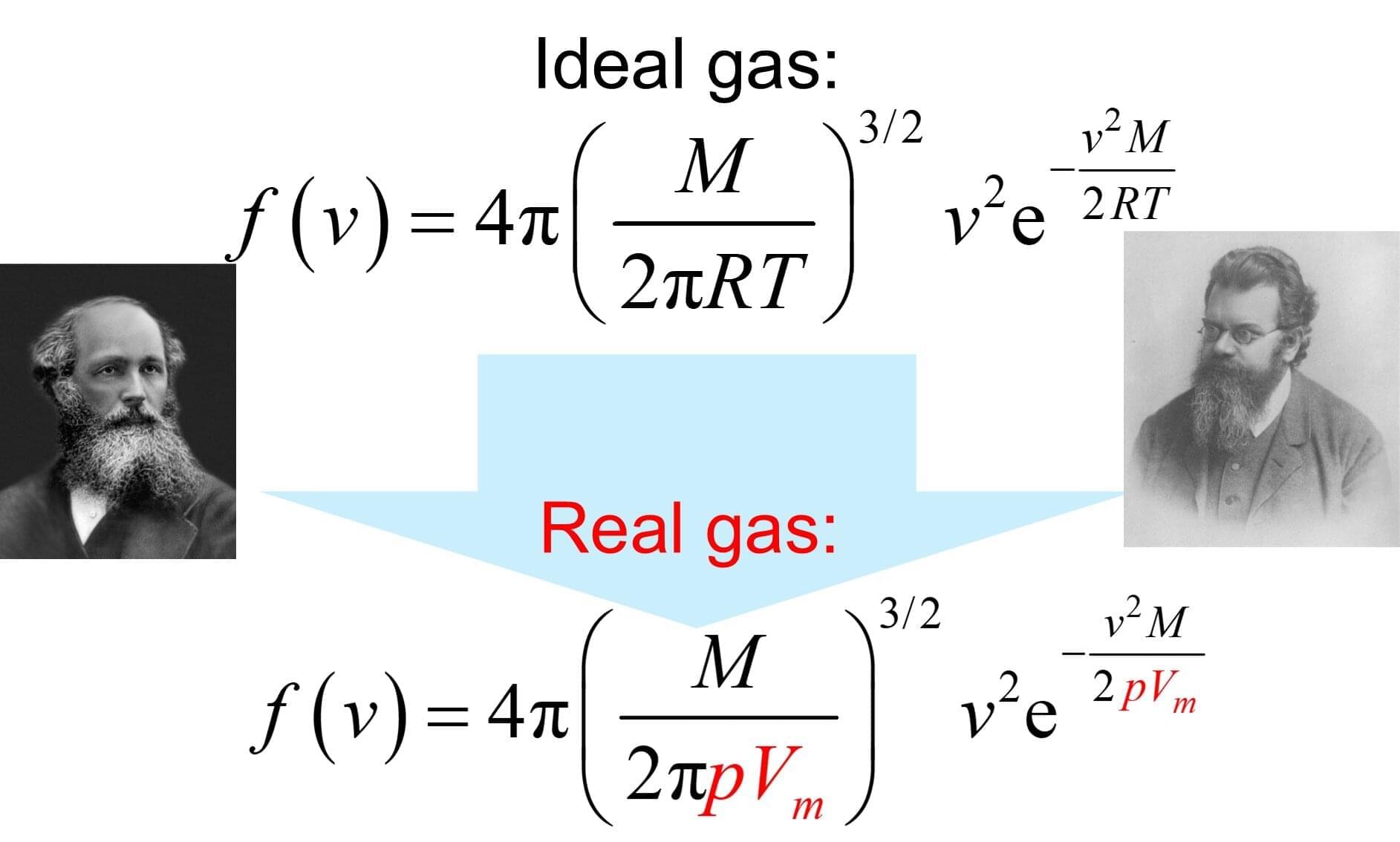
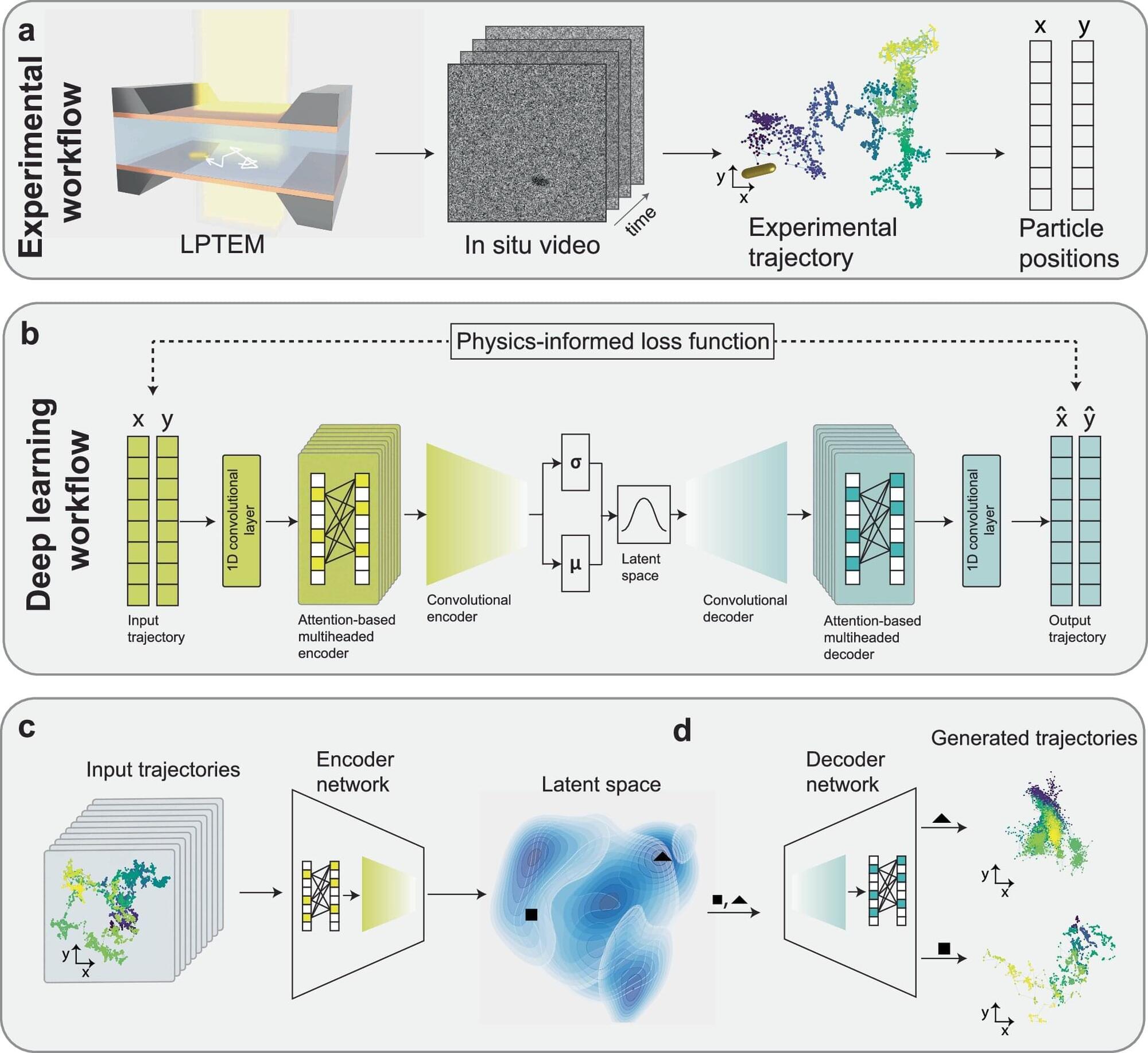
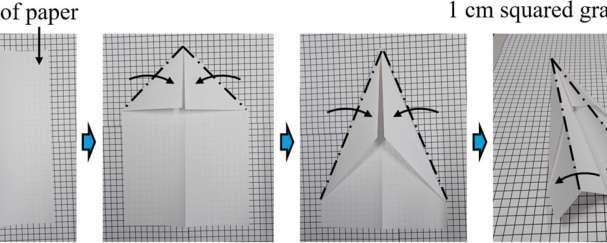
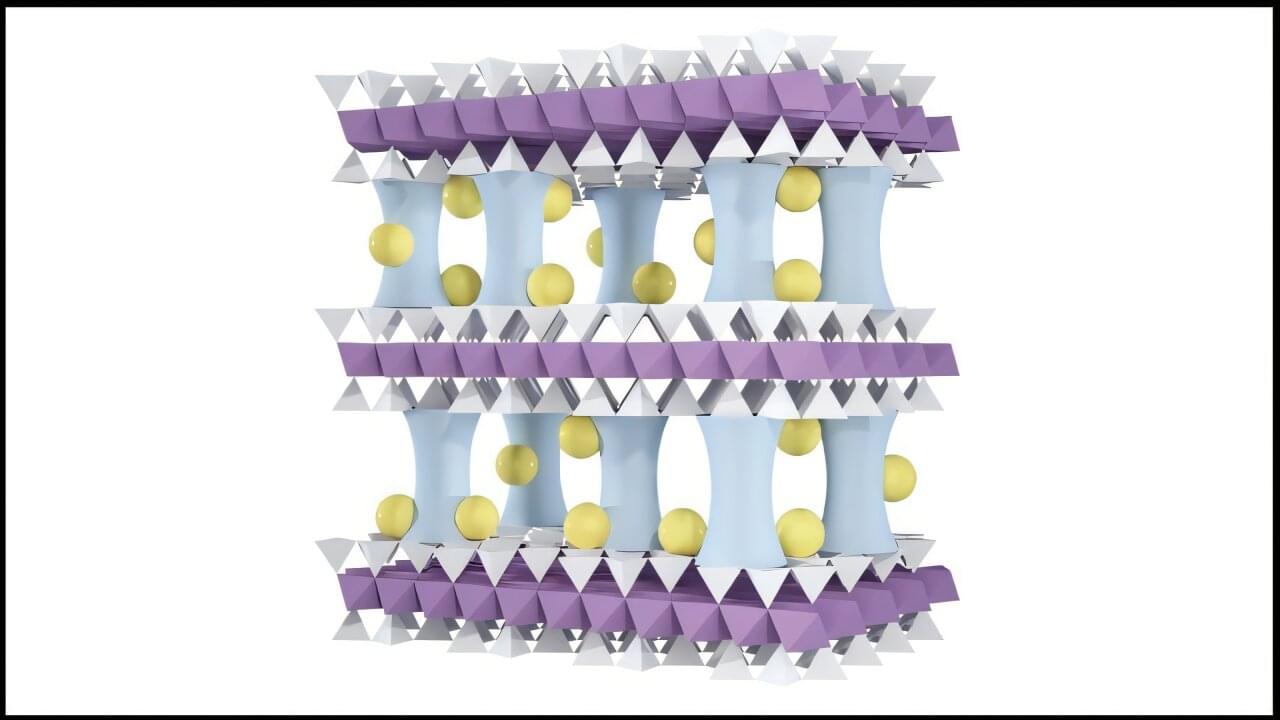
Researchers at EPFL and Kyoto University have created a stable hydrogen-rich liquid formed by mixing two simple chemicals. This breakthrough could make hydrogen storage easier, safer, and more efficient at room temperature.
Hydrogen can be the clean fuel of the future, but getting it from the lab to everyday life isn’t simple. Most hydrogen-rich materials are solids at room temperature, or they only become liquids under extreme conditions like high pressure or freezing temperatures.
Even materials such as ammonia borane, a solid, hydrogen-rich compound that can store a lot of hydrogen, are difficult because they release hydrogen only when heated, often producing unwanted byproducts.