Though participation in sports can have positive impacts both physiologically and socially, extreme sports, like football and roller derby, come with elevated risks. In a 2019 study, over 40% of 498 athletes suffered at least one injury over the course of the year.
These injury rates are even higher in elite cricket—around 70%, with about 13% of all injuries being to the head, neck, and face—pointing to a need for improvements in protective helmets.
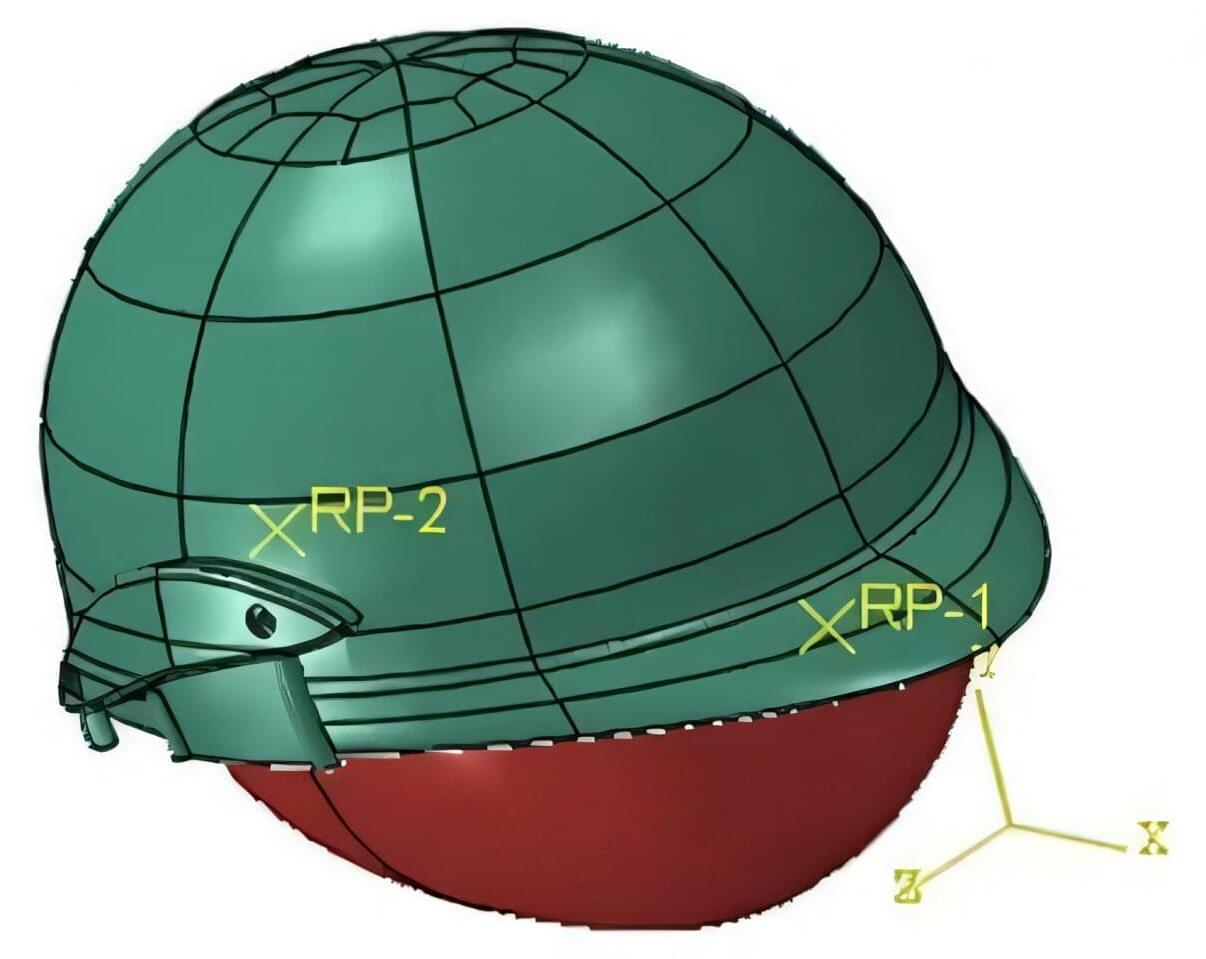
In AIP Advances, researchers from Chongqing Jiaotong University and Chongqing No. 7 Middle School compared the performance of three helmet materials under the most common types of impact and loading conditions.