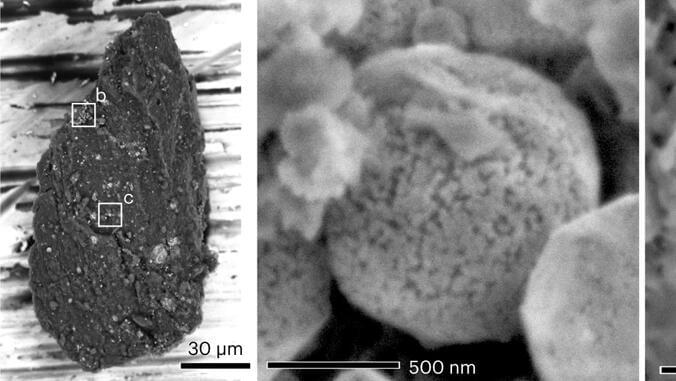
It really is impressive how many unknowns there are about the next decade in transportation. Sure, there have always been innovations and surprises, but to be unsure what most vehicles will even be powered by in 10 years, nor who — or what — will be in the driver’s seat, is astounding. Battery-electric vehicles are the leading contender to usurp internal combustion, eventually, though the road to that outcome is full of hurdles. Solid-state batteries (SSB) are seen as one of the key innovations to get there, various makers saying they’ll have at least one product with a solid-state battery on the market by the end of the decade. The overall numbers of SSB-powered vehicles might remain surprisingly low well into the 2030s, though. In Toyota’s internal news outlet, Toyota Times, the automaker wrote, “In the [SSB] mass production phase anticipated for 2030 and beyond, the companies are looking to boost capacity to several thousand tonnes (several tens of thousands of vehicles) in line with Toyota’s product plans.”
The “companies” referred to are Toyota and Japan’s petrochemical conglomerate Idemitsu Kosan, which formalized collaboration on SSBs this year. Right now, Toyota and Idemitsu are working on the development times for solid electrolyte and resulting quality and cost. When those are locked in, the firms will work on a pilot facility for commercialization. Initial commercial effort will take two years of testing and validation before wider production commences in 2030.
The “several tens of thousands of vehicles” appears to have gone through at least one revision after publication. In Jalopnik’s writeup, the capacity was quoted as “over ten thousand vehicles.” Even at the larger sum, that’s considerably less than onlookers expected, but that might be because onlookers expected too much, not because Toyota overpromised. The automaker’s talked big numbers for BEV sales, but has talked just as bigly about what kinds of electrified powertrains those sales will entail: At least four kinds of battery technologies, plus hydrogen, and hybrids. In 2021, Toyota said it expected to have an SSB ready by 2025. In 2022, a Toyota engineer said the first product to get an SSB would be a hybrid on go on sale in the first half of the decade.