Upgraded laser in California will produce one million X-ray pulses per second to study ultrafast processes at the atomic level.
Category: chemistry – Page 147
By John Jeffay, ISRAEL21c
Researchers in Israel have announced a breakthrough in safely detecting landmines – using bacteria.
They’ve developed tiny pellet-sized biosensors based on E. coli. The biosensors are dispersed over the target area, where they sniff out the chemical signature of buried explosives and become luminescent.
A team of scientists led by researchers from the University of Leicester has determined that genes responsible for learning, memory, aggression, and other complex behaviors emerged approximately 650 million years ago.
The research spearheaded by Dr. Roberto Feuda, of the Neurogenetic group within the Department of Genetics and Genome Biology, in collaboration with colleagues from the University of Leicester and the University of Fribourg (Switzerland), has recently been published in the journal Nature Communications.
<em>Nature Communications</em> is a peer-reviewed, open-access, multidisciplinary, scientific journal published by Nature Portfolio. It covers the natural sciences, including physics, biology, chemistry, medicine, and earth sciences. It began publishing in 2010 and has editorial offices in London, Berlin, New York City, and Shanghai.
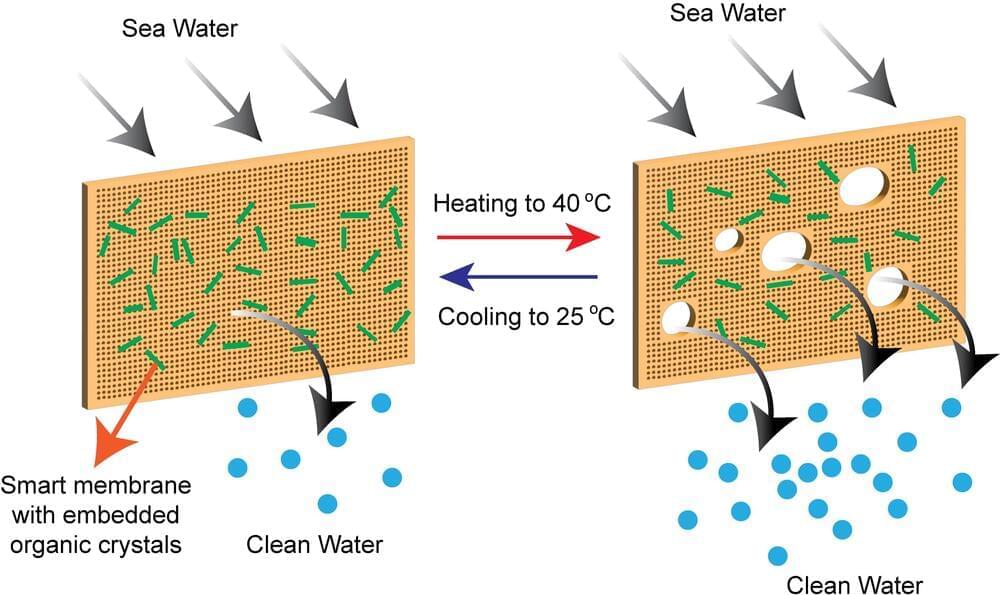
A team of NYU Abu Dhabi (NYUAD) researchers has developed a new kind of self-cleaning, hybrid membrane that provides a solution that overcomes significant challenges that have, until now, limited desalination technologies.
The most energy-efficient desalination technologies are based on membrane desalination. However, the membranes used for desalination are prone to fouling, the accumulation of scale that results in decreased membrane performance, shorter lifespan, and the need for chemical cleaning, which has unknown environmental consequences.
Researchers at NYUAD’s Smart Materials Lab and the Center for Smart Engineering Materials, led by Professor Panče Naumov and Research Scientist Ejaz Ahmed, together with their collaborators from the Institute for Membrane Technology in Italy, created a unique hybrid membrane by utilizing stimuli-responsive materials, thermosalient organic crystals, embedded in polymers. The thermosalient crystals are a new class of dynamic materials that are capable of sudden expansion or motion upon heating or cooling.
The concurrent analysis of two measurements of a biochemical signaling network can provide more information than two separate probes of the datasets.
Many biological processes depend on chemical reactions that are localized in space and time and therefore require catalytic components that self-organize. The collective behavior of these active particles depends on their chemotactic movement—how they sense and respond to chemical gradients in the environment. Mixtures of such active catalysts generate complex reaction networks, and the process by which self-organization emerges in these networks presents a puzzle. Jaime Agudo-Canalejo of the Max Planck Institute for Dynamics and Self-Organization, Germany, and his colleagues now show that the phenomenon of self-organization depends strongly on the network topology [1]. The finding provides new insights for understanding microbiological systems and for engineering synthetic catalytic colloids.
In a biological metabolic network, catalysts convert substrates into products. The product of one catalyst species acts as the substrate for another species—and so on. Agudo-Canalejo and his team modeled a three-species system. First, building on a well-established continuum theory for catalytically active species that diffuse along chemical gradients, they showed that systems where each species responds chemotactically only to its own substrate cannot self-organize unless one species is self-attracting. Next, they developed a model that allowed species to respond to both their substrates and their products. Pair interactions between different species in this more complex model drove an instability that spread throughout the three-species system, causing the catalysts to clump together. Surprisingly, this self-organization process occurred even among particles that were individually self-repelling.
The researchers say that their discovery of the importance of network topology—which catalyst species affect and are affected by which substrates and products—could open new directions in studies of active matter, informing both origin-of-life research and the design of shape-shifting functional structures.
X-ray free-electron lasers (XFELs) first came into existence two decades ago. They have since enabled pioneering experiments that “see” both the ultrafast and the ultrasmall. Existing devices typically generate short and intense x-ray pulses at a rate of around 100 x-ray pulses per second. But one of these facilities, the Linac Coherent Light Source (LCLS) at the SLAC National Accelerator Laboratory in California, is set to eclipse this pulse rate. The LCLS Collaboration has now announced “first light” for its upgraded machine, LCLS-II. When it is fully up and running, LCLS-II is expected to fire one million pulses per second, making it the world’s most powerful x-ray laser.
The LCLS-II upgrade signifies a quantum leap in the machine’s potential for discovery, says Robert Schoenlein, the LCLS’s deputy director for science. Now, rather than “demonstration” experiments on simple, model systems, scientists will be able to explore complex, real-world systems, he adds. For example, experimenters could peer into biological systems at ambient temperatures and physiological conditions, study photochemical systems and catalysts under the conditions in which they operate, and monitor nanoscale fluctuations of the electronic and magnetic correlations thought to govern the behavior of quantum materials.
The XFEL was first proposed in 1992 to tackle the challenge of building an x-ray laser. Conventional laser schemes excite large numbers of atoms into states from which they emit light. But excited states with energies corresponding to x-ray wavelengths are too short-lived to build up a sizeable excited-state population. XFELs instead rely on electrons traveling at relativistic speed through a periodic magnetic array called an undulator. Moving in a bunch, the electrons wiggle through the undulator, emitting x-ray radiation that interacts multiple times with the bunch and becomes amplified. The result is a bright x-ray beam with laser coherence.
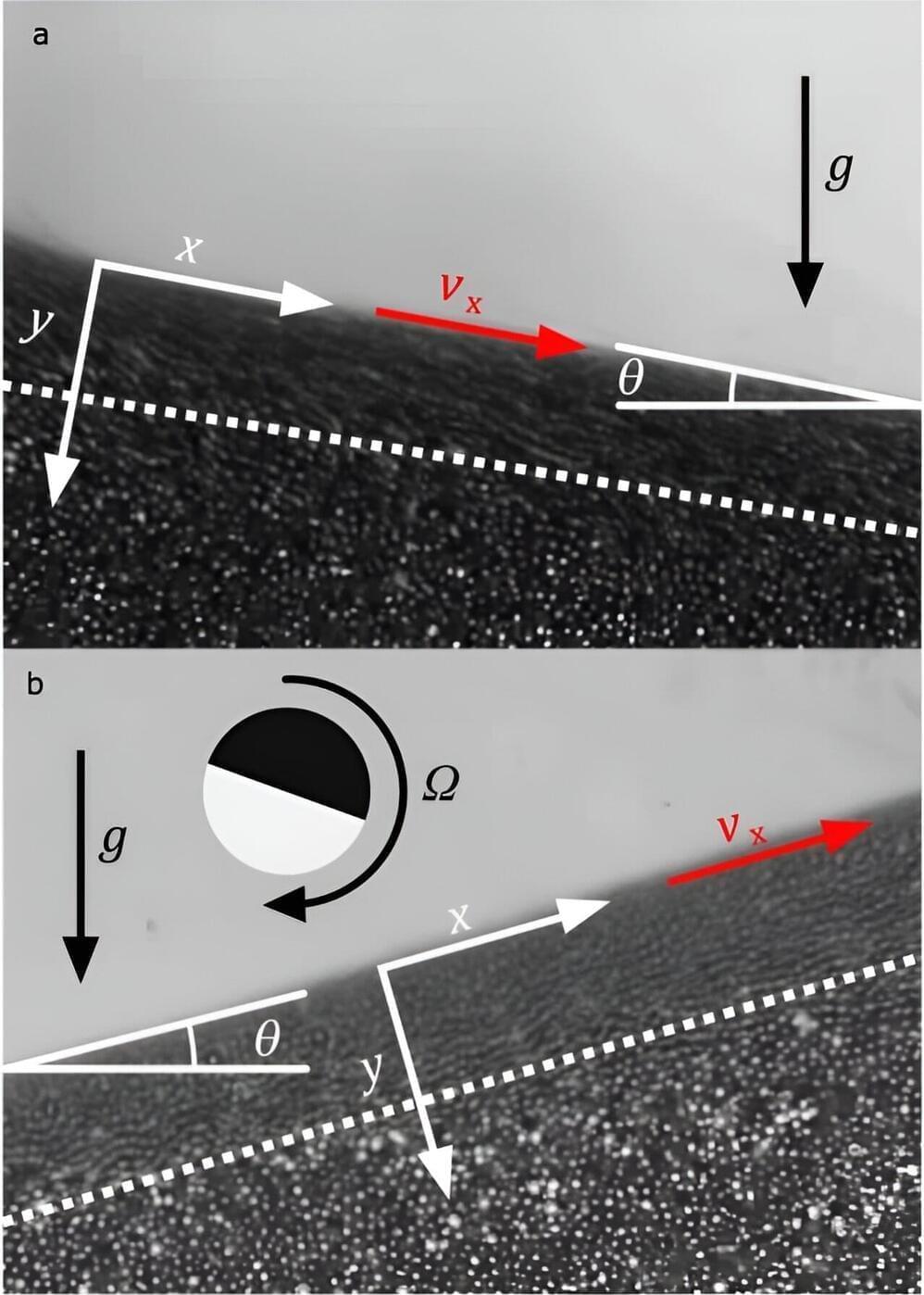
Engineering researchers at Lehigh University have discovered that sand can actually flow uphill.
The team’s findings were published today in the journal Nature Communications. A corresponding video shows what happens when torque and an attractive force is applied to each grain—the grains flow uphill, up walls, and up and down stairs.
“After using equations that describe the flow of granular materials,” says James Gilchrist, the Ruth H. and Sam Madrid Professor of Chemical and Biomolecular Engineering in Lehigh’s P.C. Rossin College of Engineering and Applied Science and one of the authors of the paper, “we were able to conclusively show that these particles were indeed moving like a granular material, except they were flowing uphill.”
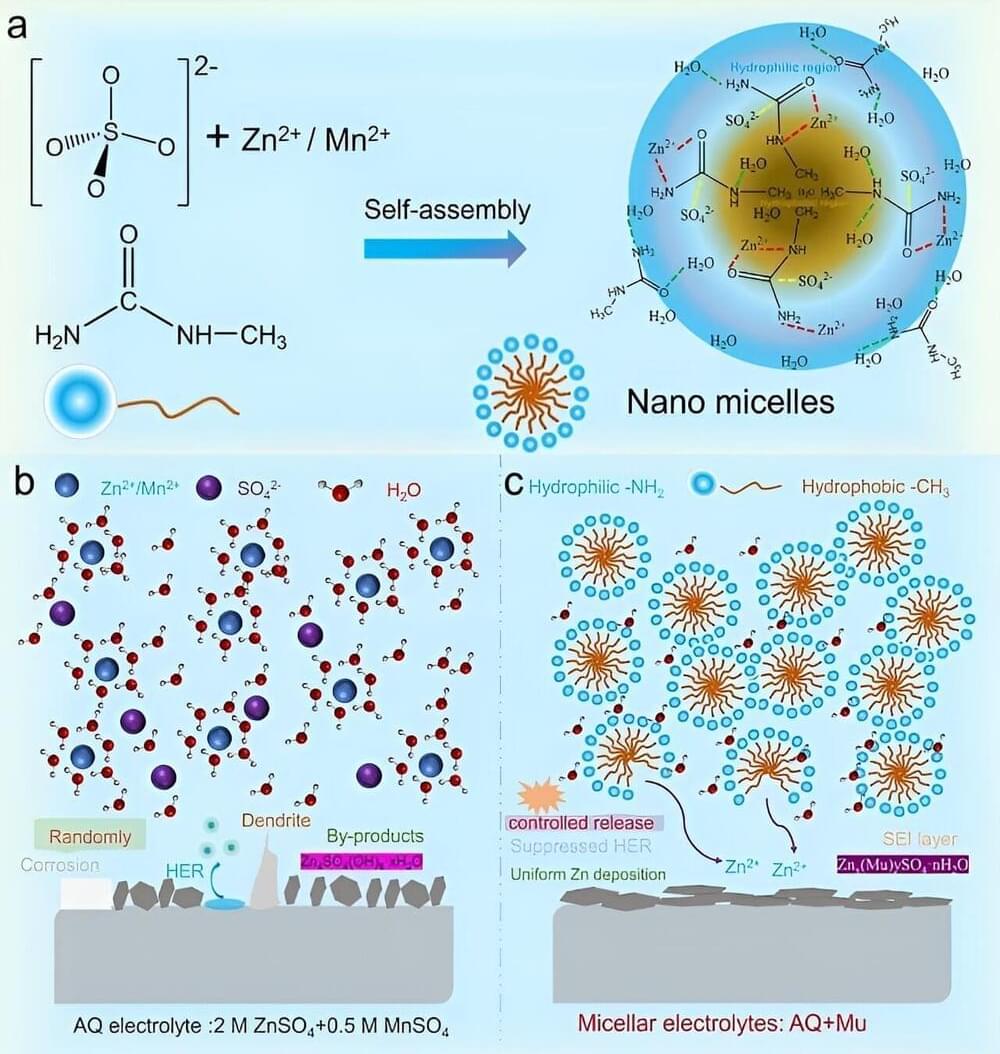
A research team led by Prof. Yan Lifeng from the University of Science and Technology of China (USTC) of the Chinese Academy of Sciences (CAS) has designed a water-based nanomicellar electrolyte by using methylurea (Mu). The results were published in the Journal of the American Chemical Society.
Aqueous zinc ion batteries (AZIBs) are competitive candidates for clean energy storage, but they are severely limited by the irreversible electrochemical reaction of the zinc anode. Therefore, it is a crucial issue to explore how to regulate the electrochemical performance of AZIBs through electrolyte design optimization.
In this paper, the researchers proposed a unique design of nanomicellar electrolyte, which comprises ZnSO4, MnSO4 and a high concentration of Mu molecules through a self-assembly strategy, where the aqueous-solvent environment is partitioned into hydrophilic and hydrophobic regions, and cations and anions are encapsulated into nanodomains.
The intricate interplay of gene expression within living cells is akin to a well-orchestrated symphony, with each gene playing its part in perfect harmony to ensure cells function as they should. At the heart of this symphony are transcription factors (TFs), molecular maestros that regulate the expression of genes by binding to specific DNA sequences known as promoters.
Unlocking the secrets of these genome-scale regulatory networks requires a comprehensive collection of gene expression profiles, but measuring gene expression responses for every TF and promoter pair has posed a formidable challenge due to the sheer number of potential combinations, even in relatively simple organisms such as bacteria.
To tackle this challenge, researchers led by Fuzhong Zhang, professor of energy, environmental & chemical engineering in the McKelvey School of Engineering at Washington University in St. Louis, developed a technique called pooled promoter responses to TF perturbation sequencing (PPTP-seq).