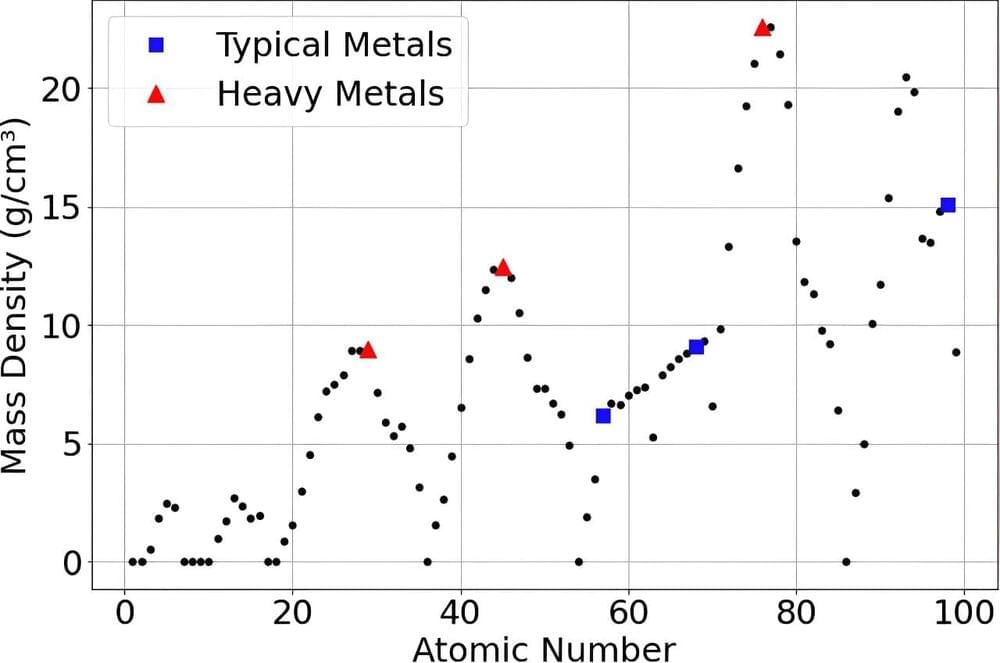
“The surprising thing we found is that in a particular kind of crystal lattice, where electrons become stuck, the strongly coupled behavior of electrons in d atomic orbitals actually act like the f orbital systems of some heavy fermions,” said Qimiao Si, co-author of a study about the research in Science Advances
<em> Science Advances </em> is a peer-reviewed, open-access scientific journal that is published by the American Association for the Advancement of Science (AAAS). It was launched in 2015 and covers a wide range of topics in the natural sciences, including biology, chemistry, earth and environmental sciences, materials science, and physics.