😗
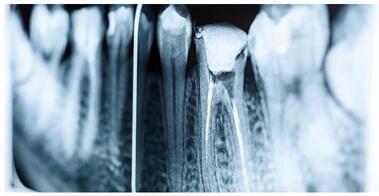
Root canal treatment removes the infection and bacteria from the core of a tooth — the pulp chamber. These bacteria are often present within the canals of the teeth. However, proper treatment saves a badly infected natural tooth from needing to be extracted. Sufficient cleaning of the root canals is a key step of RCT. A lack of proper canal debridement can cause bacteria to thrive — a significant cause of RCT failures.
The tooth is washed with antibiotics or other chemicals that kill the bacteria to get rid of the infection. However, some teeth have complex root structures, and conventional ways of cleaning them are not enough to remove all bacteria. That’s one area where dental nanorobots can help. Nanorobots are showing promise in different steps of RCT, even better than traditional ways.
Dental nanorobots, also called nanobots/nanomotors/nano propellers, are designed to reach nooks and crannies within teeth to disinfect even the narrowest and most complex tooth canals during RCT. As the name suggests, nanorobots are microscopic — one-millionth of a millimeter. Dentists need special equipment like electron microscopes to see them. Their tiny size helps them to enter tooth canals and maneuver to depths and through curves not previously accessible.