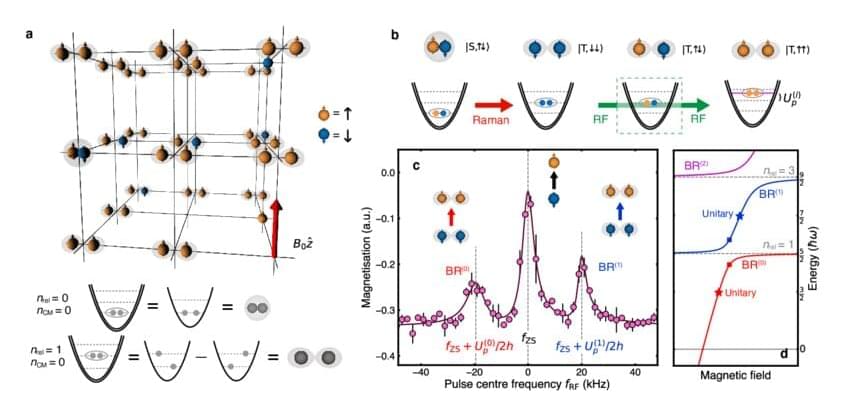
“Suppose you knew everything there was to know about a water molecule—the chemical formula, the bond angle, etc.,” says Joseph Thywissen, a professor in the Department of Physics and a member of the Centre for Quantum Information & Quantum Control at the University of Toronto.
“You might know everything about the molecule, but still not know there are waves on the ocean, much less how to surf them,” he says. “That’s because when you put a bunch of molecules together, they behave in a way you probably cannot anticipate.”
Thywissen is describing the concept in physics known as emergence: the relationship between the behavior and characteristics of individual particles and large numbers of those particles. He and his collaborators have taken a first step in understanding this transition from “one-to-many” particles by studying not one, not many, but two isolated, interacting particles, in this case potassium atoms.