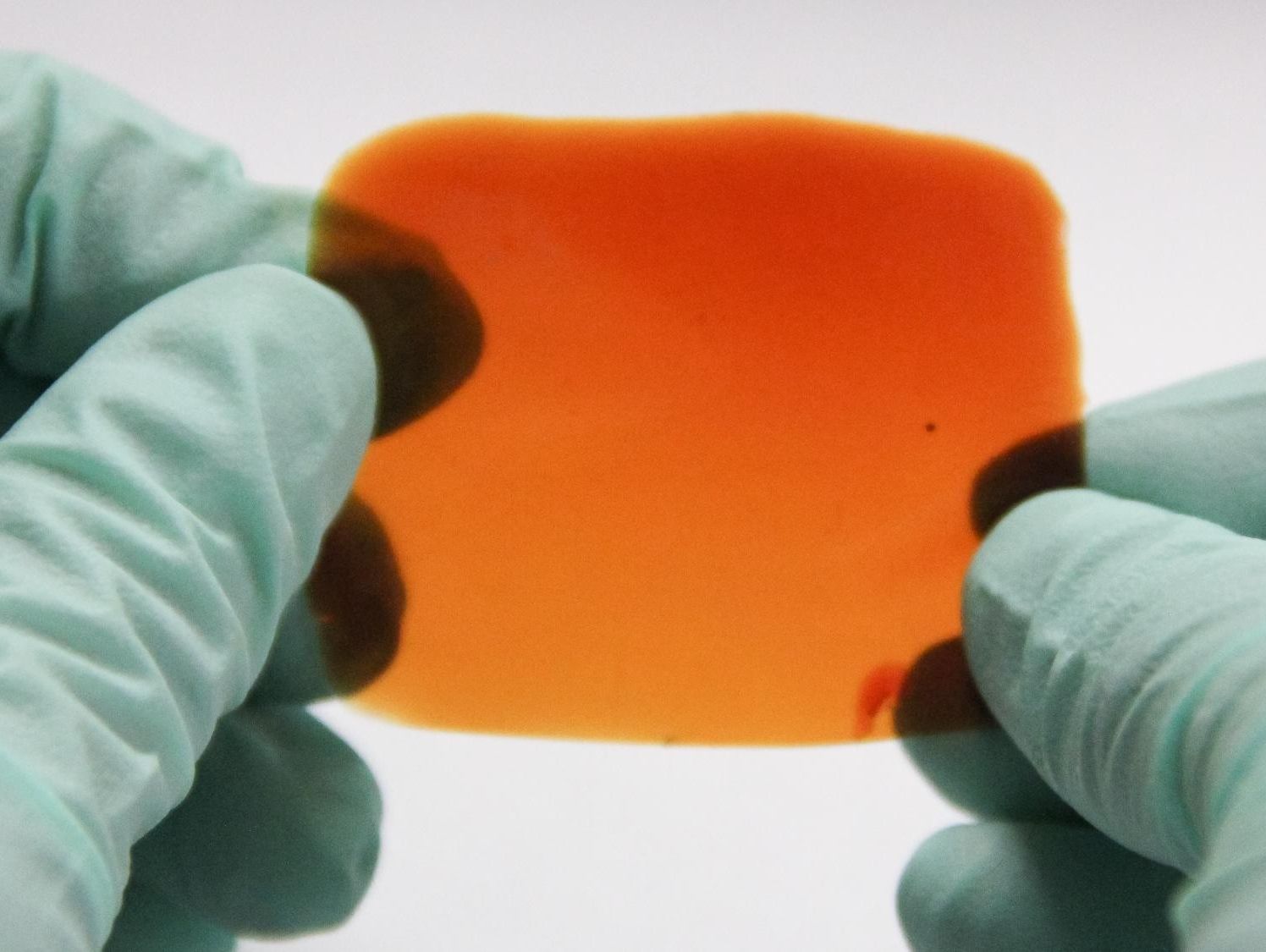
(Phys.org)—Animal muscle needs to be strong enough to endure strain; it must also be flexible and elastic; and it is self-healing. Finding a polymer that has all of these properties has proved challenging. However, researchers from Stanford, Nanjing University, UC Riverside, Harvard, and the University of Colorado have reported the synthesis of an elastomer that mimics the properties of animal muscle. Their polymer, is also stable at room temperature and not sensitive to water. Their work appears in Nature Chemistry.
Efforts to create polymers that mimic the properties of biological muscle have come short of being practically useful. Often the bonding involved in making these polymers must be sufficiently strong to serve as actuators, but weak enough for reversible self-healing. Many models, to date, involve hydrogen bonding, but hydrogen bonds are sensitive to water. Li, et al. have, instead, exploited metal-ligand interactions as a way to mimic muscle properties.
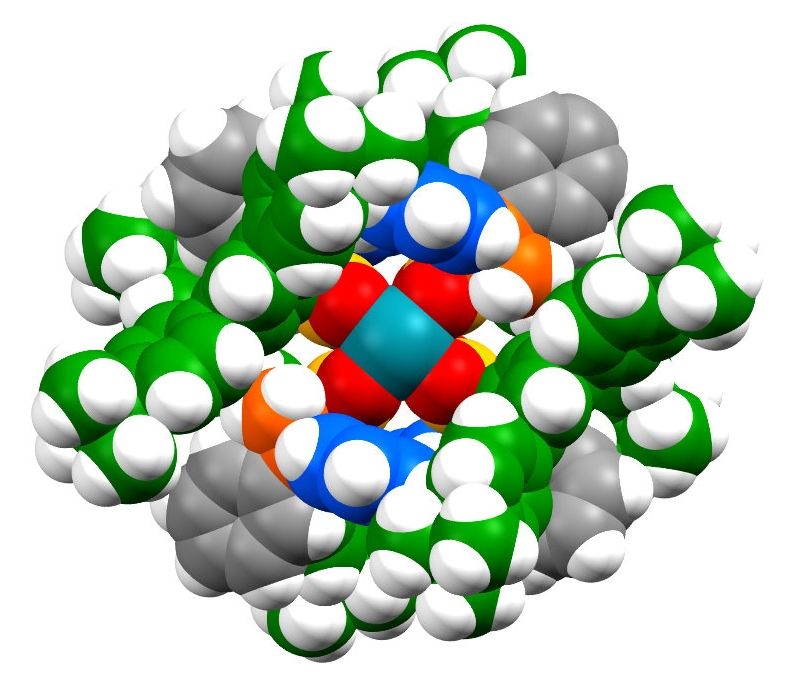
The ligand 2,6-pyridinedicarboxamide (pdca)binds to Fe(III) via the pyridyl nitrogen and the nitrogen and oxygen on the carboxamides. Two pdca molecules coordinate to one Fe(III) atom through six coordination sites. Two of the sites are strong bonds (the pyridyl), two sites are “medium” strength bonds (the amides), and two are weak bonds (the carboxyl). Calculations of bond strength show that the strong bonds are similar to covalent bonds, while the weak Fe-O bonds are similar to hydrogen bonding. This multi-bonding structure, as it turns out, provides an excellent framework for making an elastomer.
Read more