Apr 15, 2008
$153 million/city thin film plastic domes can protect against nuclear weapons and bad weather
Posted by Brian Wang in categories: biological, chemistry, defense, existential risks, habitats, lifeboat, military, nanotechnology, nuclear weapons, sustainability
Cross posted from Nextbigfuture
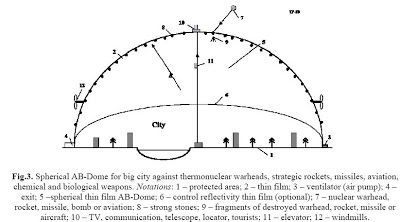
Now Alexander Bolonkin has come up with a cheaper, technological easy and more practical approach with thin film inflatable domes. It not only would provide protection form nuclear devices it could be used to place high communication devices, windmill power and a lot of other money generating uses. The film mass covered of 1 km**2 of ground area is M1 = 2×10**6 mc = 600 tons/km**2 and film cost is $60,000/km**2.
The area of big city diameter 20 km is 314 km**2. Area of semi-spherical dome is 628 km2. The cost of Dome cover is 62.8 millions $US. We can take less the overpressure (p = 0.001atm) and decrease the cover cost in 5 – 7 times. The total cost of installation is about 30–90 million $US. Not only is it only about $153 million to protect a city it is cheaper than a geosynchronous satellite for high speed communications. Alexander Bolonkin’s website