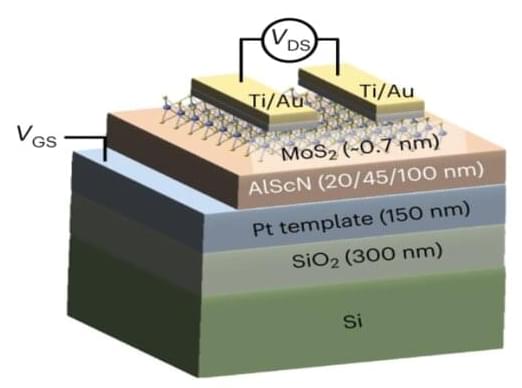
Summary: Researchers have uncovered genes essential for learning, memory, aggression, and other complex behaviors originated around 650 million years ago.
The study utilized computational methods to trace the evolutionary history of these genes involved in the production, modulation, and reception of monoamines like serotonin, dopamine, and adrenaline. This discovery suggests that this new method of modulating neuronal circuits could have played a role in the Cambrian Explosion, contributing to the diversification of life.
The finding offers new research avenues to understand the origins of complex behaviors and their relation to diverse processes like reward, addiction, aggression, feeding, and sleep.