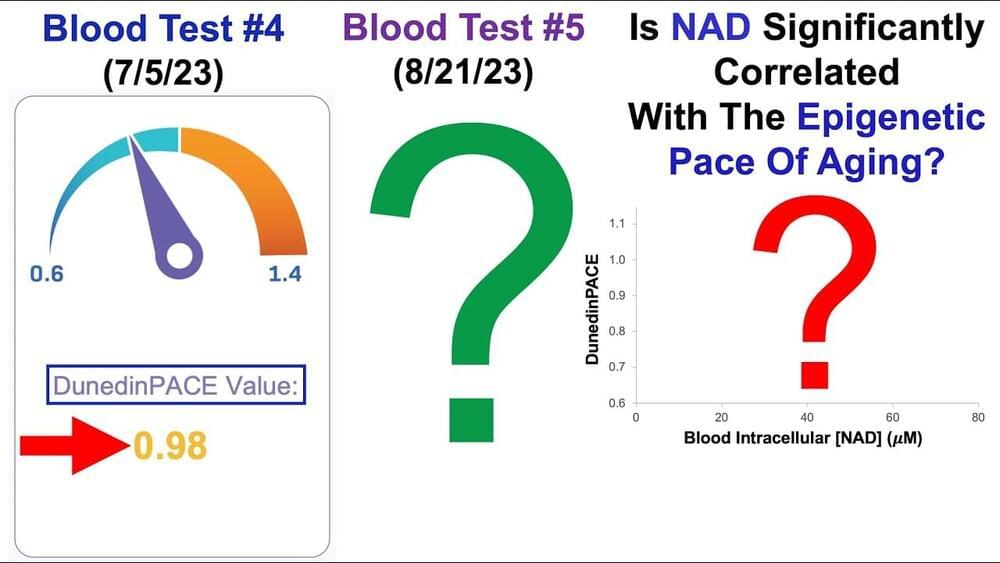
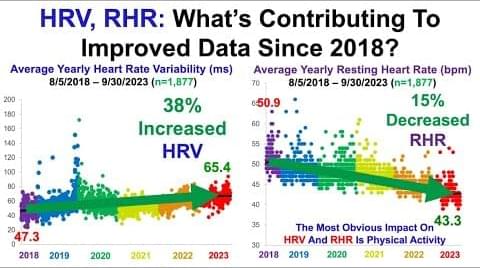
This is a bit technical. “nucleocytoplasmic compartmentalization assay”, Yeah buddy.
Life is dependent on the preservation and storage of information. The genome and epigenome are the two central storehouses of information in eukaryotes, and although they work interdependently, they are fundamentally quite different. Genetic information is consistent across all body cells throughout the life of an individual while epigenetic information varies between cells as well as changes over time and as per environment.
Researchers have identified several hallmarks of aging such as epigenetic alterations, genomic instability, cellular senescence, telomere attrition, mitochondrial dysfunction, and others [1]. These are known to play a role in the dysfunction and deterioration of cells with age. David Sinclair and other researchers have previously indicated that loss of epigenetic information can cause changes in gene expression, leading to cellular identity loss. Previous studies in mice have also shown that cell injuries such as cell crushing and DNA double-strand breaks can promote loss of epigenetic information which can accelerate aging along with age-related diseases [2].
Cellular senescence is a state of stable cell cycle arrest that can be triggered due to a wide range of extrinsic as well as intrinsic factors. It promotes tissue remodeling, wound repair, and cancer prevention by stopping the proliferation of damaged and aged cells. Senescent cells are characterized by metabolic and morphological alterations, reorganization of the chromatin, and release of pro-inflammatory substances known as the senescence-associated secretory phenotype (SASP) [3]. Irreparable DNA damage, loss of epigenetic information, and telomere shortening are a few factors that can initiate cellular senescence. Accumulation of senescent cells with age results in inflammation as well as the generation of reactive oxygen species (ROS).