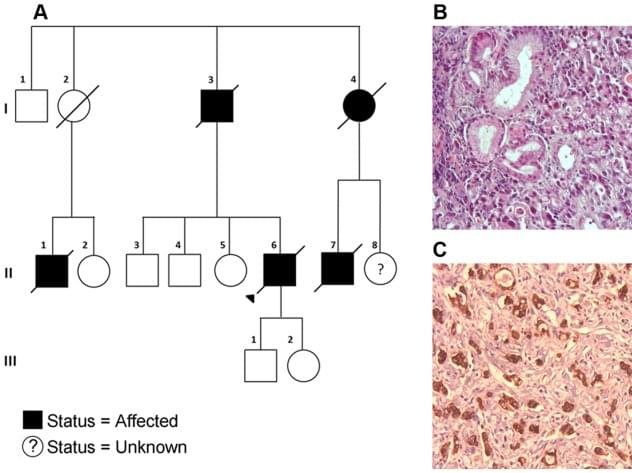
Like biomarker tests, germline testing can help doctors determine the best treatments for patients, but such testing may also help identify people whose family members should be offered testing for potential cancer-causing gene changes.
Guidelines recommend that germline testing be offered to all people with male breast cancer, ovarian cancer, pancreatic cancer, and metastatic prostate cancer. For other cancers with lower likelihood of harmful inherited mutations, recommendations for germline testing vary.
But new findings from a study that is examining the extent of testing for germline mutations among people diagnosed with cancer in California and Georgia between 2013 and 2019 found that germline testing rates are still low. Among the more than 1.3 million people in the study, only about 93,000, or 6.8%, underwent germline genetic testing through March 31, 2021, according to findings published July 3 in JAMA.