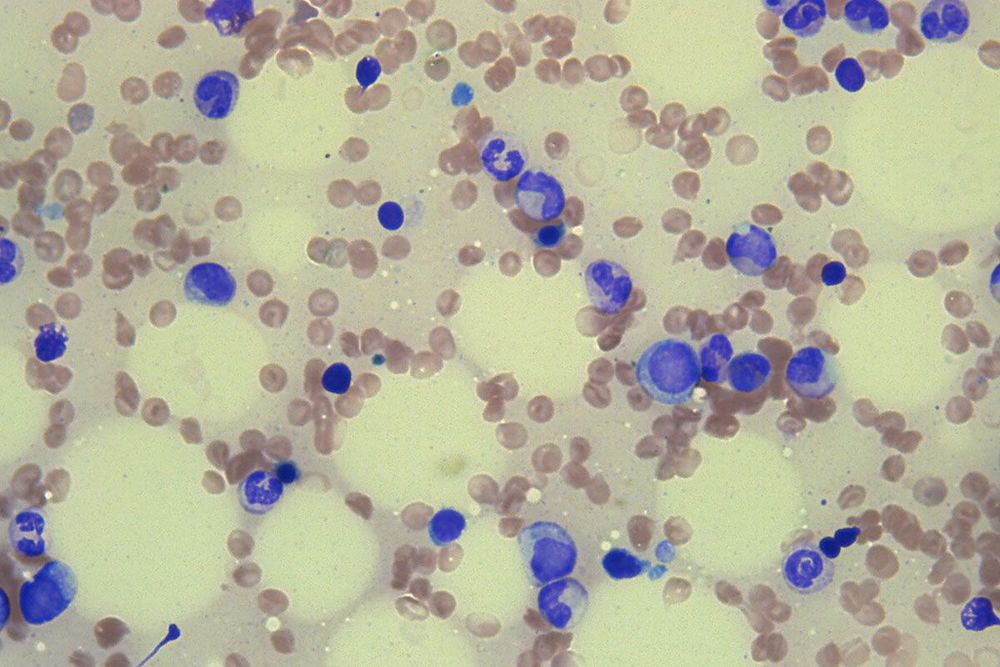
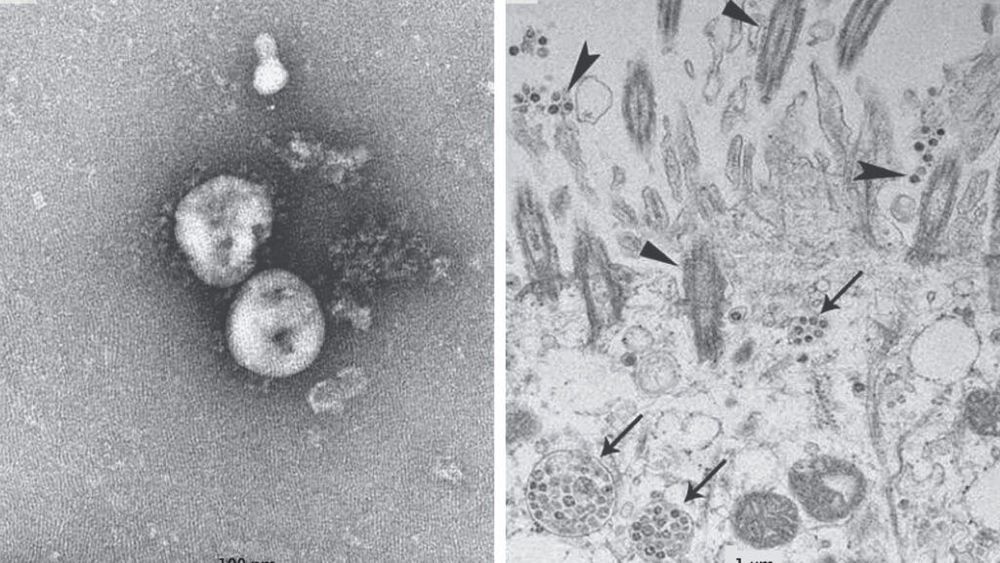
Circa 2017 Bats harbor a large diversity of coronaviruses (CoVs), several of which are related to zoonotic pathogens that cause severe disease in humans. Our screening of bat samples collected in Kenya from 2007 to 2010 not only detected RNA from several novel CoVs but, more significantly, identified sequences that were closely related to human CoVs NL63 and 229E, suggesting that these two human viruses originate from bats. We also demonstrated that human CoV NL63 is a recombinant between NL63-like viruses circulating in Triaenops bats and 229E-like viruses circulating in Hipposideros bats, with the breakpoint located near 5′ and 3′ ends of the spike (S) protein gene. In addition, two further interspecies recombination events involving the S gene were identified, suggesting that this region may represent a recombination “hot spot” in CoV genomes. Finally, using a combination of phylogenetic and distance-based approaches, we showed that the genetic diversity of bat CoVs is primarily structured by host species and subsequently by geographic distances.
bMarie Bashir Institute for Infectious Diseases and Biosecurity, Charles Perkins Centre, School of Life and Environmental Sciences and Sydney Medical School, The University of Sydney, Sydney, Australia.
Find articles by Mang Shi
aDivision of Viral Diseases, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.




 We are delighted to announce that
We are delighted to announce that