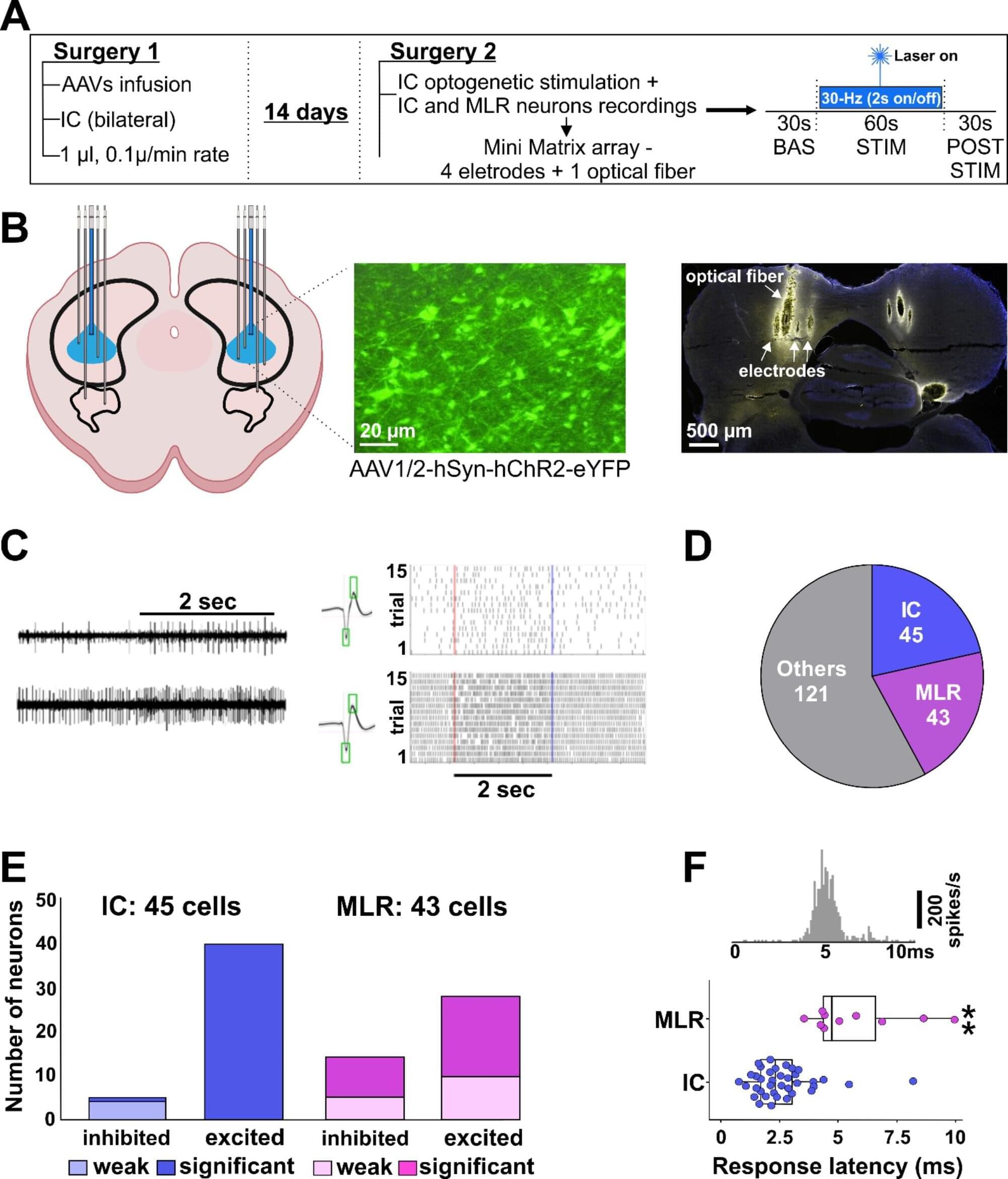
Learning and motivation are driven by internal and external rewards. Many of our day-to-day behaviours are guided by predicting, or anticipating, whether a given action will result in a positive (that is, rewarding) outcome. The study of how organisms learn from experience to correctly anticipate rewards has been a productive research field for well over a century, since Ivan Pavlov’s seminal psychological work. In his most famous experiment, dogs were trained to expect food some time after a buzzer sounded. These dogs began salivating as soon as they heard the sound, before the food had arrived, indicating they’d learned to predict the reward. In the original experiment, Pavlov estimated the dogs’ anticipation by measuring the volume of saliva they produced. But in recent decades, scientists have begun to decipher the inner workings of how the brain learns these expectations. Meanwhile, in close contact with this study of reward learning in animals, computer scientists have developed algorithms for reinforcement learning in artificial systems. These algorithms enable AI systems to learn complex strategies without external instruction, guided instead by reward predictions.
The contribution of our new work, published in Nature (PDF), is finding that a recent development in computer science – which yields significant improvements in performance on reinforcement learning problems – may provide a deep, parsimonious explanation for several previously unexplained features of reward learning in the brain, and opens up new avenues of research into the brain’s dopamine system, with potential implications for learning and motivation disorders.
Reinforcement learning is one of the oldest and most powerful ideas linking neuroscience and AI. In the late 1980s, computer science researchers were trying to develop algorithms that could learn how to perform complex behaviours on their own, using only rewards and punishments as a teaching signal. These rewards would serve to reinforce whatever behaviours led to their acquisition. To solve a given problem, it’s necessary to understand how current actions result in future rewards. For example, a student might learn by reinforcement that studying for an exam leads to better scores on tests. In order to predict the total future reward that will result from an action, it’s often necessary to reason many steps into the future.