Oct 25, 2023
New design solves stability and efficiency of perovskite solar cells
Posted by Saúl Morales Rodriguéz in categories: solar power, sustainability
Researchers at EPFL and Northwestern University have unveiled a groundbreaking design for perovskite solar cells, creating one of the most stable PSCs with a power-conversion efficiency above 25%, paving the way for future commercialization.
Perovskite solar cells (PSCs) stand at the forefront of solar energy innovation, and have drawn a lot of attention for their power-conversion efficiency and cost-effective manufacturing. But the way to commercialization of PSCs still has a hurdle to overcome: achieving both high efficiency and long-term stability, especially in challenging environmental conditions.
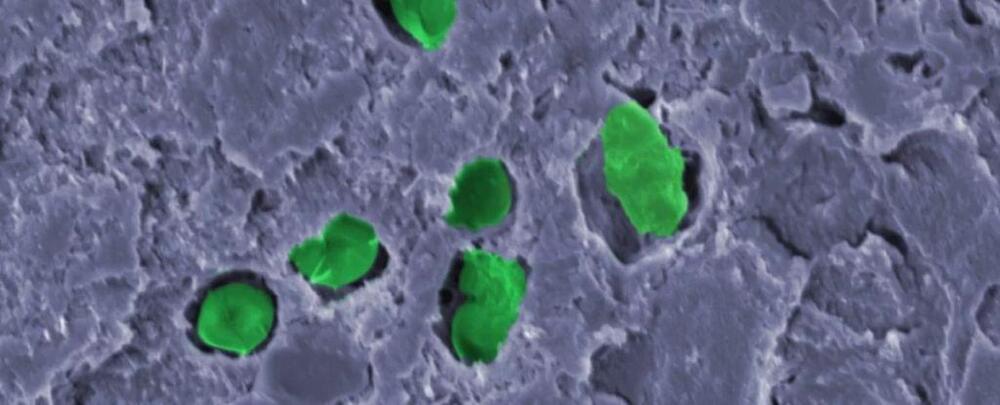
The solution lies in the interplay between the layers of PSCs, which has proven to be a double-edged sword. The layers can enhance the cells’ performance but also cause them to degrade too quickly for regular use in everyday life.