These guys have a great idea…but In true Zuckerberg style how does one steal and supercharge the idea. With food having salmonella, people need to grow more food at home. What technology can be created that uses technology to help people in urban settings grow their own food. This will help many in a post covid world, and the food should be safer, and also may promote nutrition. nnAmerican farmers also are having trouble, and would see the loss in demand. Global food production needs to increase. Japan offered to boost the continent of Africa’s rice production through cooperation. The same cooperation needs to be done with American farmers to boost Africa’s food production. Technology would be used to partner American farmers with African village cooperatives. The farmers and cooperatives would work together and share profits. This way the American farmer has revenue coming from two markets and continents. The same model can also be used in Mexico to prevent immigration. This way American farmers would also have revenue coming from Central and South America, however people who normally would be farm workers would be partners, and make more than they would having to cross borders dangerously, to make less money. This model can both reduce poverty, as well and insure food security. The capital for investment would have to come from many sources. Crowdfunding is one that can be good as the money can be paid back with profit. This way a crowd fund investment would gain better returns than interest rates. The next of course would be USAID. A project can be developed, in which USAID provides American farmers with start up capital. They manage the project pay back the loans, while sharing profits. Agreements can be developed for certain periods of time, After which the American farmer turns the project over to the cooperatives…just thinking out of the box it is a bit crazy. The farmers would be like a new Peace Corps thing. #VillageEconomics nnPortfolio company #ApolloAgriculture was recently featured in a Forbes article highlighting their machine-learning and automated-operations technology that helps small-scale farmers access everything they need to maximize their profitability. #impactinvesting #agtech
Between 2011 and 2014, engineer and Stanford grad Eli Pollak worked in agricultural technology in the U.S. for a company called the Climate Corporation. The enterprise where he was one of the early employees (which in 2013 was acquired by Monsanto for over $1 billion) worked on providing customized recommendations to increase production of large scale commercial farmers. What caught Pollak’s eye during his tenure at the company, however, was that some countries were planting way more seeds, but producing dramatically less agricultural products than the U.S.
This prompted Pollak to team up with Climate Corporation colleague Earl St Sauver, and Benjamin Ngenga (who himself grew up on a farm) to start Apollo Agriculture, a Kenyan ag-tech company which uses machine learning and automated operations technology to help small-scale farmers access everything they need to maximize their profitability.
Continue reading “Meet The Agritech Entrepreneurs Who Just Raised $6 Million To Help Farmers In Kenya Grow Their Business” »
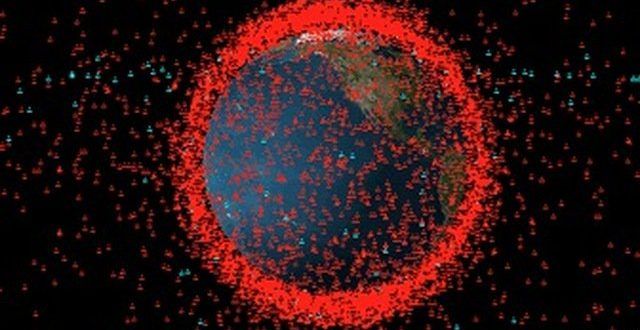
 As part of the partnership between SpaceWatch. Global and Joint Air Power Competence Centre, we have been granted permission to publish selected articles and texts. We are pleased to present “Space Traffic Management – Impact of Large Constellations on Military Operations in Space”, originally published by the Joint Air Power Competence Centre for the Conference Read Ahead 2020.
As part of the partnership between SpaceWatch. Global and Joint Air Power Competence Centre, we have been granted permission to publish selected articles and texts. We are pleased to present “Space Traffic Management – Impact of Large Constellations on Military Operations in Space”, originally published by the Joint Air Power Competence Centre for the Conference Read Ahead 2020.