Archive for the ‘sustainability’ category: Page 597
Aug 3, 2016
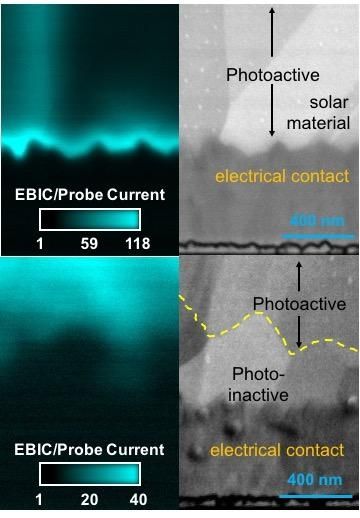
ORNL optimizes formula for cadmium-tellurium solar cells
Posted by Karen Hurst in categories: solar power, sustainability
Nice and Kudos to ORNL.
A team led by Jonathan Poplawsky of the Center for Nanophase Materials Sciences used advanced microscopy techniques to discover efficiency differences of crystalline structures of various mixtures of cadmium, tellurium and selenium. In fact, selenium is an integral part of the formulation that resulted in a world record for solar cell efficiency. The team’s paper is published in Nature Communications.
While some of today’s solar cells use a blend of cadmium and tellurium to convert light into electricity, adding the optimum amount of selenium in the right places could help increase efficiency from the current mark of about 22 percent to levels approaching the theoretical limit of 30–33 percent. The trick is to determine the best ratio of selenium.
Continue reading “ORNL optimizes formula for cadmium-tellurium solar cells” »
Aug 3, 2016
Foreign rail firms shunted as ‘Made in China’ mantra gathers pace
Posted by Karen Hurst in categories: energy, government, sustainability
Made in China motto is gaining speed in China.
SHANGHAI Foreign firms say they are struggling to gain access to China’s vast railway market as the country, seeking to transform its domestic industry into an export powerhouse, tightens the bidding criteria on rail tenders.
The complaints echo similar concerns raised in other industries including technology and renewable energy, and highlight what some foreign companies see as an uneven playing field when operating in China.
Continue reading “Foreign rail firms shunted as ‘Made in China’ mantra gathers pace” »
Aug 3, 2016
Tesla acquires sister firm SolarCity for $2.6 billion
Posted by Shailesh Prasad in categories: Elon Musk, solar power, sustainability, transportation
Tesla buys SolarCity.
(Reuters) — SolarCity Corp agreed to be acquired by sister company Tesla Motors Inc in a deal worth $200 million less than the initial offer, sending shares of both companies down in early trading on Monday.
Electric vehicle maker Tesla expects to achieve “significant” cost savings and “dramatic improvements” in manufacturing efficiency as a result of the acquisition of solar panel installer SolarCity, Tesla Chief Executive Officer Elon Musk said on Monday.
Continue reading “Tesla acquires sister firm SolarCity for $2.6 billion” »
Aug 2, 2016
Tesla is building an electric minibus based on the Model X
Posted by Andreas Matt in categories: climatology, economics, Elon Musk, energy, robotics/AI, sustainability
Elon Musk has been a busy man lately as he works to transition the world to renewable energy and sustainable transportation with the goal of decarbonizing the global economy to meet the challenge of climate change. To meet that goal, Tesla will need to address “high passenger-density urban transport” – and Musk just confirmed plans to create a fully autonomous electric Minibus using the Model X chassis.
Aug 1, 2016
Elon Musk is kicking off an automated low-carbon future with the merger of Tesla and SolarCity
Posted by Klaus Baldauf in categories: Elon Musk, robotics/AI, sustainability, transportation
Elon Musk is today set to merge Tesla Motors and SolarCity, Reuters is reporting, kicking off part two of his master plan to transform our cities and suburbs into environmentally friendly automated wonderlands.
In July Musk wrote of his plan to merge the two companies in a blog post entitled Master Plan, Part Deux, saying it was essential to “create a smoothly integrated and beautiful solar-roof-with-battery product that just works, empowering the individual as their own utility, and then scale that throughout the world.
“We can’t do this well if Tesla and SolarCity are different companies, which is why we need to combine and break down the barriers inherent to being separate companies. Now that Tesla is ready to scale Powerwall and SolarCity is ready to provide highly differentiated solar, the time has come to bring them together.”
Jul 31, 2016
Lab 2.0: Will Computers Replace Experimental Science?
Posted by Karen Hurst in categories: chemistry, computing, mobile phones, physics, science, solar power, sustainability
We spend our lives surrounded by hi-tech materials and chemicals that make our batteries, solar cells and mobile phones work. But developing new technologies requires time-consuming, expensive and even dangerous experiments.
Luckily we now have a secret weapon that allows us to save time, money and risk by avoiding some of these experiments: computers.
Continue reading “Lab 2.0: Will Computers Replace Experimental Science?” »
Jul 31, 2016
Artificial Intelligence May Soon Drive Your Car — And Keep You Company at the Same Time
Posted by Aleksandar Vukovic in categories: robotics/AI, sustainability, transportation
Honda said in a press release that the AI will use conversations with the driver and other data it gathers ‘both to perceive the emotions of the driver and to engage in dialogue with the driver based on the vehicle’s own emotions.’ The just-announced partnership works toward application of the ‘emotion engine,’ which is ‘a set of AI technologies developed by cocoro SB Corp., which enable machines to artificially generate their own emotions.’
Image source: Getty Images.
Continue Reading Below.
Jul 29, 2016
Smart bricks will transform how buildings work
Posted by Karen Hurst in categories: computing, engineering, habitats, sustainability
Smart bricks capable of recycling wastewater and generating electricity from sunlight are being developed by a team of scientists from the University of the West of England (UWE Bristol). The bricks will be able to fit together and create ‘bioreactor walls’ which could then be incorporated in housing, public building and office spaces.
The UWE Bristol team is working on the smart technologies that will be integrated into the bricks in this pan European ‘Living Architecture’ (LIAR) project led by Newcastle University. The LIAR project brings together living architecture, computing and engineering to find a new way to tackle global sustainability issues.
The smart living bricks will be made from bio-reactors filled with microbial cells and algae. Designed to self-adapt to changing environmental conditions the smart bricks will monitor and modify air in the building and recognise occupants.
Jul 29, 2016
PV Nano Cell’s nanometric silver conductive ink enables non-contact digital inkjet printing
Posted by Karen Hurst in categories: solar power, sustainability
Nice.
PV Nano Cell has commercially developed ‘Sicrys’, a single-crystal, nanometric silver conductive ink delivering enhanced performance for digital conductive printing in mass production applications. The inks are also available in copper-based form, delivering all of the product’s properties and advantages with improved cost efficiency.
Problem