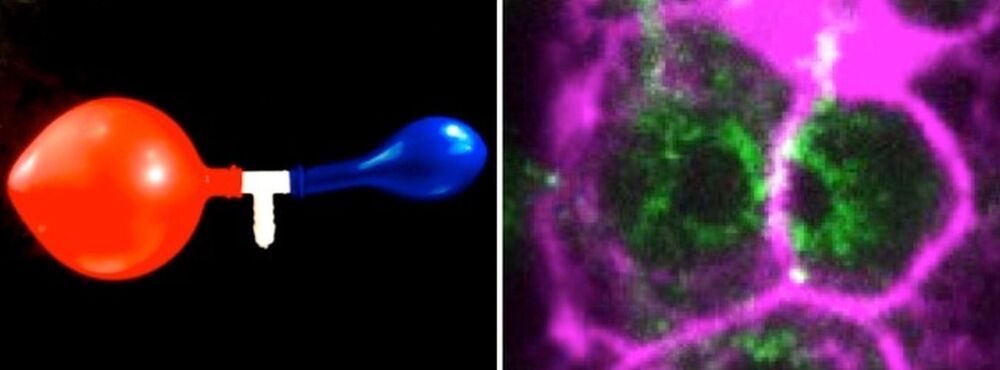
In many species including humans, the cells responsible for reproduction, the germ cells, are often highly interconnected and share their cytoplasm. In the hermaphrodite nematode Caenorhabditis elegans, up to 500 germ cells are connected to each other in the gonad, the tissue that produces eggs and sperm. These cells are arranged around a central cytoplasmic “corridor” and exchange cytoplasmic material fostering cell growth, and ultimately produce oocytes ready to be fertilized.
In past studies, researchers have found that C. elegans gonads generate more germ cells than needed and that only half of them grow to become oocytes, while the rest shrink and die by physiological apoptosis, a programmed cell death that occurs in multicellular organisms. Now, scientists from the Biotechnology Center of the TU Dresden (BIOTEC), the Max Planck Institute of molecular Cell Biology and Genetics (MPI-CBG), the Cluster of Excellence Physics of Life (PoL) at the TU Dresden, the Max Planck Institute for the Physics of Complex Systems (MPI-PKS), the Flatiron Institute, NY, and the University of California, Berkeley, have found evidence to answer the question of what triggers this cell fate decision between life and death in the germline.
Prior studies revealed the genetic basis and biochemical signals that drive physiological cell death, but the mechanisms that select and initiate apoptosis in individual germ cells remained unclear. As germ cells mature along the gonad of the nematode, they first collectively grow in size and in volume homogenously. In the study just published in Nature Physics, the scientists show that this homogenous growth suddenly shifts to a heterogenous growth where some cells become bigger and some cells become smaller.








Comments are closed.