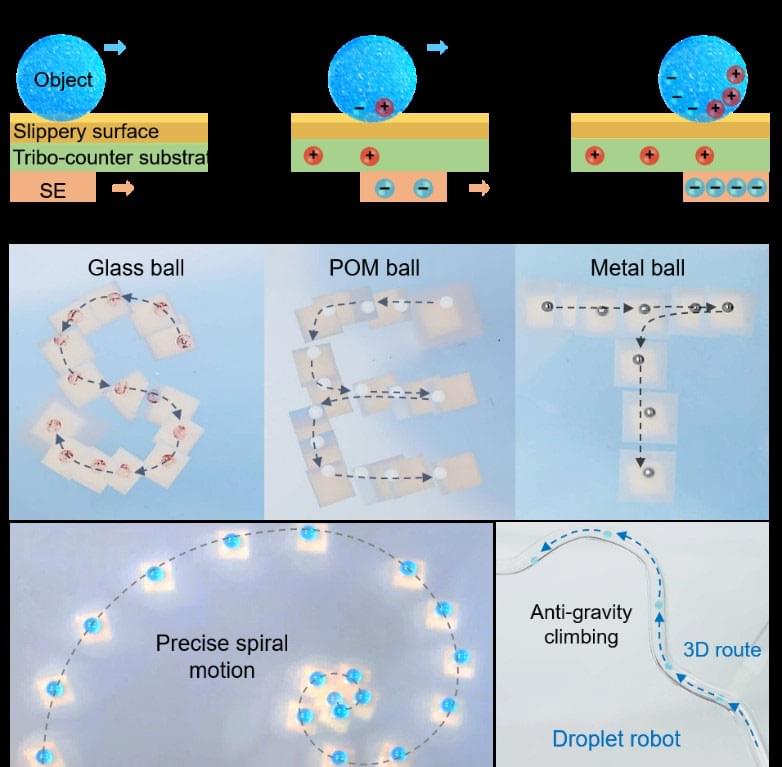
In a study published in Device (“Self-powered electrostatic tweezer for adaptive object manipulation”), a research team led by Dr. DU Xuemin from the Shenzhen Institute of Advanced Technology (SIAT) of the Chinese Academy of Sciences has reported a new self-powered electrostatic tweezer that offers superior accumulation and tunability of triboelectric charges, enabling unprecedented flexibility and adaptability for manipulating objects in various working scenarios.
The ability to manipulate objects using physical tweezers is essential in fields such as physics, chemistry, and biology. However, conventional tweezers often require complex electrode arrays and external power sources, have limited charge-generation capabilities, or produce undesirable temperature rises.
The newly proposed self-powered electrostatic tweezer (SET) features a polyvinylidene fluoride trifluoroethylene (P(VDF-TrFE))-based self-powered electrode (SE) that generates large and tunable surface charge density through the triboelectric effect, along with a dielectric substrate that functions as both a tribo-counter material and a supportive platform, and a slippery surface to reduce resistance and biofouling during object manipulation.